Biomedical Engineering Reference
In-Depth Information
RNAi probably evolved initially in primitive organisms in order to protect their genomes from viruses,
transposons and additional insertable genetic elements, and to regulate gene expression. The RNAi path-
way was fi rst discovered in plants, but it is now known to function in most, if not all, eukaryotes.
RNAi represents a sequence-specifi c post-translational inhibition mechanism of gene expres-
sion, induced ultimately by dsRNA, be it produced naturally or synthesised
in vitro
and introduced
into a cell. Entry of dsRNA triggers its cleavage into short (21-23 nucleotide long) sequences
called short interfering RNAs (siRNAs). This cleavage is catalysed by a cellular nuclease enzyme
called 'Dicer'. The siRNA is incorporated into a multi-subunit effector complex known as an RNA-
induced silencing complex (RISC), which also contains several nucleic acid processing enzymes
(a helicase, an endonuclease and an exonuclease). The double-stranded siRNA then unwinds (a
process promoted by the helicase activity), and the 'sense' strand of the dsRNA is discarded.
The remaining 'antisense' siRNA strand then facilitates RISC binding to a specifi c mRNA via
Watson-Crick base complementarity, which is then degraded by RISC nuclease activity.
RNAi technology has obvious therapeutic potential as an antisense agent, and initial therapeutic tar-
gets of RNAi include viral infection, neurological diseases and cancer therapy. The synthesis of dsRNA
displaying the desired nucleotide sequence is straightforward. However, as in the case of additional
nucleic-acid-based therapeutic approaches, major technical hurdles remain to be overcome before RNAi
becomes a therapeutic reality. Naked unmodifi ed siRNAs for example display a serum half-life of less
than 1 min, due to serum nuclease degradation. Approaches to improve the RNAi pharmacokinetic
profi le include chemical modifi cation of the nucleotide backbone, to render it nuclease resistant, and the
use of viral or non-viral vectors, to achieve safe product delivery to cells. As such, the jury remains out in
terms of the development and approval of RNAi-based medicines, in the short to medium term at least.
Certain RNA sequences can function as catalysts. These so-called ribozymes function to cata-
lyse cleavage at specifi c sequences in a specifi c mRNA substrate. Many ribozymes will cleave
their target mRNA where there exists a particular triplet nucleotide sequence G-U-C. Statisti-
cally, it is likely that this triplet will occur at least once in most mRNAs.
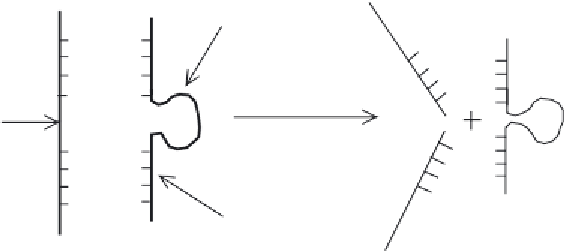
Ribozymes can be directed to a specifi c mRNA by introducing short fl anking oligonucleotides
that are complementary to the target mRNA (Figure 14.16). The resultant cleavage of the target
Target
mRNA
Cleaved mRNA
Free
ribozyme
Ribozyme
A
C
C
G
U
G
G
C
Nucleotide
sequence
at which
ribozyme
can cleave
C
G
G
C
G
C
A
U
Flanking sequences
which 'dock' ribozyme
at the appropriate
sequence of the
appropriate mRNA via
complementary base
pairing
Figure 14.16
Outline of how ribozyme technology could prevent translation of specifi c mRNA, thus preventing
synthesis of a specifi c target protein
Search WWH ::

Custom Search