Biomedical Engineering Reference
In-Depth Information
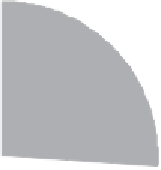
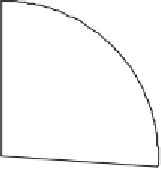
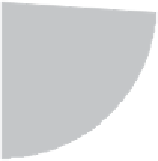
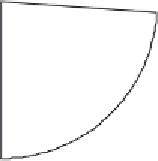
Adenovirus (26%)
Retrovirus (24%)
Naked/Plasmid DNA (17%)
Lipofection (8%)
Others (25%)
Figure 14.2
Vectors used thus far in gene therapy trials. 'Others' are mainly viral-based and include the use
of pox, vaccinia and adeno-associated viruses, as well as herpes simplex virus. Data adapted from www.wiley.
co.uk/genemed/clinical
Target cells for
gene therapy
Vector
Target cells for
gene theraphy
(a)
(c)
(b)
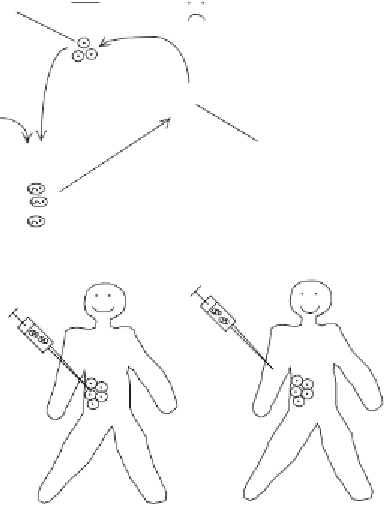
Figure 14.3
The various practical approaches that may be pursued when undertaking gene therapy. (a)
In
vitro
gene therapy entails removal of target cells from the body followed by their incubation with nucleic
acid-containing vector. After the vector delivers the nucleic acid into the human cells, they are placed back
in the body. (b)
In situ
gene therapy entails direct injection of the vector immediately adjacent to the body
target cells. (c)
In vivo
gene therapy involves intravenous administration of the vector. The vector has been
designed such that it will only recognize and bind the intended target cells. In this way, the nucleic acid is
delivered exclusively to those cells. Refer to text for further details






Search WWH ::

Custom Search