Biomedical Engineering Reference
In-Depth Information
to Epstein-Barr virus infection. Even upon successful transformation, most produce low-affi nity
IgM antibodies, and the cells are often unstable. Having said that, one monoclonal antibody ap-
proved for medical use (Humaspect, Table 13.2) is produced by a human lymphoblastoid cell line
originally transformed by Epstein-Barr virus.
Fusion of human lymphocytes with human lymphoblastoid cell lines is a very ineffi cient
process. Fusion of human lymphocytes with murine myeloma cells lead to very unstable hybrids.
Upon fusion, preferential loss of human genetic elements is often observed. Unfortunately, par-
ticularly common is the loss of chromosomes 2, 14 and 22, which encode antibody light and heavy
chain loci. The production yields of human monoclonals upon immortalization of the human
B-lymphocyte (by whatever means) are also low.
13.3.6 Chimaeric and humanized antibodies
Recombinant DNA technology has provided an alternative (and successful) route of reducing the
innate immunity of murine monoclonals. The genes for all human immunoglobulin sub-types
have been cloned, and this has allowed generation of various hybrid antibody structures of reduced
immunogenicity.
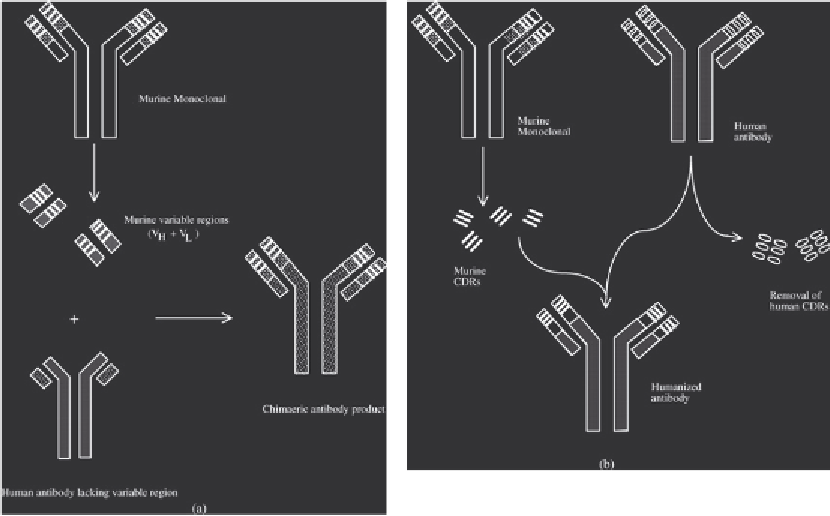
The first strategy entails production of 'chimaeric' antibodies, consisting of mouse vari-
able regions and human constant regions (Figure 13.9). The chimaeric antibody would dis-
play the specificity of the original murine antibody, but would largely be human in sequence.
Figure 13.9
Production of chimaeric (a) and humanized (b) antibodies (via recombinant DNA technology).
Chimaeric antibodies consist of murine monoclonal V
H
and V
L
domains grafted onto the F
c
region of a human
antibody. Humanized antibody consists of murine CDR regions grafted into a human antibody
Search WWH ::

Custom Search