Biomedical Engineering Reference
In-Depth Information
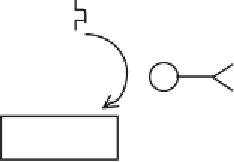
Prodrug
(inactive)
Cancer cell
E
Active drug
(cytocidal)
Key:
E
= (Prodrug activating) enzyme-antibody conjugate
= Tumor-associated antigen
Figure 13.7
Outline of antibody-directed enzyme prodrug therapy (ADEPT). Subsequent to its enzymatic
activation, the active drug is taken up by the cell, upon which it exhibits a cytocidal effect. Refer to text for
specifi c detail
for example, i.v. injection. This would subsequently be activated only at the tumour surface
(Figure 13.7). This approach has been termed ADEPT or antibody-directed catalysis.
Because of its catalytic nature, a single antibody-enzyme conjugate would activate many mole-
cules of the prodrug in question. Much of the active cytocidal agent released at the tumour surface
would be taken up by the tumour cells via simple diffusion or carrier-mediated active transport.
Administration of etoposide in prodrug form exemplifi es this approach (Figure 13.8). Etoposide
(C
29
H
32
O
13
; molecular mass 588.6) is a semi-synthetic derivative of podophyllotoxin, produced
naturally by the North American plant
Podophyllum peltatum
. It is used as an anti-cancer agent.
Its cellular uptake is diffusion dependent, and once inside the cell it exerts its cytocidal effect.
Phosphorylated etoposide is non-diffusable and, hence, represents an inactive prodrug form of
etoposide. (Attachment of a charged group to most diffusion-dependent drugs prevents their
cellular uptake.) Alkaline phosphatase, however, can cleave the phosphate group, releasing free
cytocidal agent. Administration of a tumour-detecting antibody-alkaline phosphatase conjugate
thus effectively targets the enzyme to the tumour surface. Subsequent administration of phos-
phorylated etoposide results in etoposide liberation at the tumour surface, which can then enter
tumour cells by diffusion (Figure 13.8). Various other prodrug-enzyme combinations have now
been developed, including phenoxyacetamide derivatives of doxorubicin (activated by penicillin
amidase) and 5-fl uorocytosine (activated by cytosine deaminase).
The prodrugs used should be inexpensive, readily available and should be stable to chemical/enzy-
matic degradation
in vivo
. Enzymes used should also be stable under physiological conditions, display
a reasonable turn over number
in vivo
and not be dependent upon a co-factor for activity. Mammalian
enzymes would be likely less immunogenic than microbial enzymes. However, the use of a prodrug
capable of being activated by a mammalian enzyme can lead to complications if that enzyme's (hu-
man) endogenous counterpart is capable of activating the drug at sites distant from the tumour.





Search WWH ::

Custom Search