Biomedical Engineering Reference
In-Depth Information
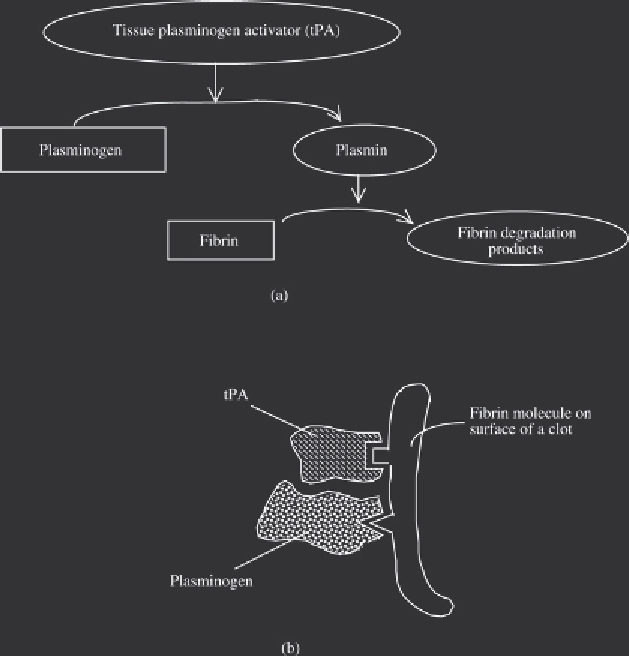
Figure 12.11
(a) The fi brinolytic system, in which tPA proteolytically converts the zymogen plasminogen into
active plasmin, which in turn degrades the fi brin strands, thus dissolving the clot. tPA and plasminogen both bind
to the surface of fi brin strands (b), thus ensuring rapid and effi cient activation of the thrombolytic process
Fibrin contains binding sites for both plasminogen and tPA, thus bringing these into close prox-
imity. This facilitates direct activation of the plasminogen at the clot surface (Figure 12.11). This
activation process is potentiated by the fact that binding of tPA to fi brin (a) enhances the sub-
sequent binding of plasminogen and (b) increases tPA's activity towards plasminogen by up to
600-fold.
Overall therefore, activation of the thrombolytic cascade occurs exactly where it is needed, i.e.
on the surface of the clot. This is important, as the substrate specifi city of plasmin is poor, and
circulating plasmin displays the catalytic potential to proteolyse fi brinogen, factor V and factor
VIII. Although soluble serum tPA displays a much reduced activity towards plasminogen, some
free-circulating plasmin is produced by this reaction. If uncontrolled, this could increase the risk
of subsequent haemorrhage. This scenario is usually averted because circulating plasmin is rap-
idly neutralized by anther plasma protein,
α
2
-antiplasmin (a 70 kDa single-chain glycoprotein that
binds plasmin very tightly in a 1:1 complex). In contrast to free plasmin, plasmin present on a clot
Search WWH ::

Custom Search