Biomedical Engineering Reference
In-Depth Information
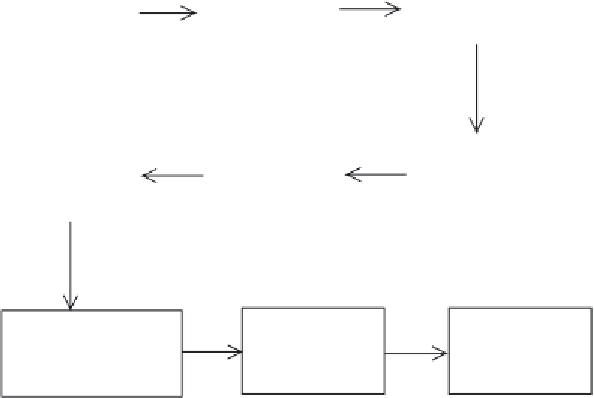
Initial chromatographic
fractionation of filtrate
by, for example, ion-
exchange chromatography
Fermentation of
Saccharmomyces
cerevisiae
Harvesting of
cells (filtration)
Concentration and salt
removal (ultrafiltration
and diafiltration)
High resolution
chromatography
Ultrafiltration
(concentration)
Addition of excipients
(mannitol) and pH
adjustment
Freeze drying of
final product
Filtration and
aseptic filling
Figure 12.9
Overview of the production of Refl udan (recombinant hirudin). The exact details of many
steps remain confi dential for obvious commercial reasons. A number of QC checks are carried out on the fi nal
product to confi rm the product's structure. These include amino acid composition, HPLC analysis and peptide
mapping
group normally present on tyrosine
63
. Clinical trials, however, have proven this slightly altered
product to be both safe and effective. The fi nal product is presented in freeze-dried form with the
sugar mannitol representing the major added excipient. The product, which displays a useful shelf-
life of 2 years when stored at room temperature, is reconstituted with saline or WFI immediately
prior to its i.v. administration. A second recombinant product (tradename revasc, also produced in
S. cerevisiae
) has also been approved.
12.3.2 Antithrombin
Antithrombin, already mentioned in the context of heparin, is the most abundantly occurring
natural inhibitor of coagulation. It is a single-chain 432 amino acid glycoprotein displaying four
oligosaccharide side chains and an approximate molecular mass of 58 kDa. It is present in plasma
at concentrations of 150
g ml
1
and is a potent inhibitor of thrombin (factor IIa), as well as of
factors IXa and Xa. It inhibits thrombin by binding directly to it in a 1:1 stoichiometric complex.
Plasma-derived antithrombin concentrates have been used medically since the 1980s for the
treatment of hereditary and acquired antithrombin defi ciency. Hereditary (genetic) defi ciency is
characterized by the presence of little/no native antithrombin activity in plasma and results in an
increased risk of inappropriate blood clot/emboli formation. Acquired antithrombin defi ciency
can be induced by drugs (e.g. heparin and oestrogens), liver disease (decreased antithrombin
µ





Search WWH ::

Custom Search