Biomedical Engineering Reference
In-Depth Information
Endotoxin
Factor C
Active Factor C
Factor B
Active Factor B
Factor A
Active Factor A
Coagulogen
Coagulin & peptide C
Polymerization
Clot
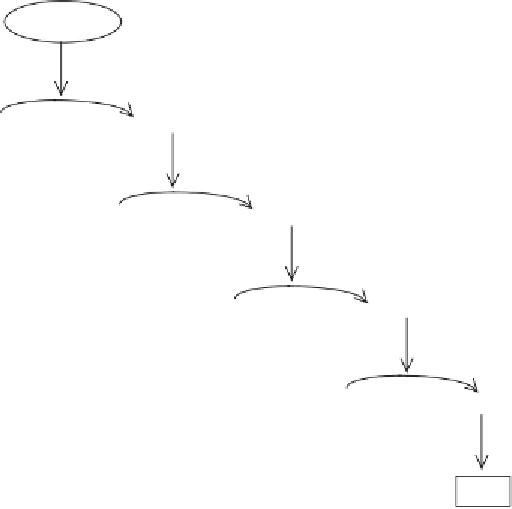
Figure 7.8
Activation of clot formation by endotoxin. The presence of endotoxin causes stepwise, sequen-
tial activation of various clotting factors present naturally within the amoebocytes of the American horse-
shoe crab. The net result is the generation of the polypeptide fragment coagulin, which polymerizes, thus
forming a gel or clot
Its major disadvantage is its selectivity: it only detects endotoxin-based pyrogens. In practice,
however, endotoxin represents the pyrogen that is by far the most likely to be present in pharma-
ceutical products. The LAL method is used extensively within the industry. It is used not only to
detect endotoxin in fi nished parenteral preparations, but also in WFI and in biological fl uids, such
as serum or cerebrospinal fl uid.
Before the LAL assay is routinely used to detect/quantify endotoxin in any product, its effec-
tive functioning in the presence of that product must be demonstrated by validation studies. Such
studies are required to prove that the product (or, more likely, excipients present in the product) do
not interfere with the rate/extent of clot formation (i.e. are neither inhibitors nor activators of the
LAL-based enzymes). LAL enzyme inhibition could facilitate false-negative results upon sample
assay. Validation studies entail, for example, observing the effect of spiking endotoxin-negative
product with know quantities of endotoxin, or spiking endotoxin with varying quantities of prod-
uct, before assay with the LAL reagents.
All ancillary reagents used in the LAL assay system (e.g. WFI, test tubes, pipette tips for liquid
transfer, etc.) must obviously be endotoxin free. Such items can be rendered endotoxin free by
heat. Its heat-stable nature, however, renders very vigorous heating necessary in order to destroy
contaminant endotoxin. A single autoclave cycle is insuffi cient, with total destruction requiring
three consecutive autoclave cycles. Dry heat may also be used (180
C for 3 h or 240
C for 1 h).
Search WWH ::

Custom Search