Biomedical Engineering Reference
In-Depth Information
denaturation at interfaces. Polysorbate, for example, is included in some
γ
-globulin preparations,
cytokines and in some monoclonal antibody-based products.
Although various polysorbates are used, the experience with an EPO-based product (trade-
name Eprex) sounds a potential cautionary note in terms of formulation development, as outlined
in Box 4.1.
6.9.3 Final product fi ll
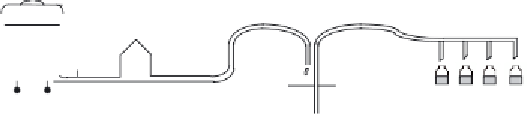
An overview of a typical fi nal product fi lling process is presented in
Figure
6.23. The bulk
fi nal product fi rst undergoes QC testing to ensure its compliance with bulk product specifi -
cations. Although implementation of good practices during manufacturing will ensure that
the product carries a low microbial load, it will not be sterile at this stage. The product
is then passed through a (sterilizing) 0.22
m fi lter (
Figure
6.24). The sterile product is
housed (temporarily) in a sterile-product holding tank, from where it is aseptically fi lled
into pre-sterile fi nal product containers (usually glass vials). The fi lling process normally
employs highly automated liquid fi lling systems. All items of equipment, pipework, etc. with
which the sterilized product comes into direct contact must obviously themselves be sterile.
Most such equipment items may be sterilized by autoclaving, and be aseptically assembled
prior to the fi lling operation (which is undertaken under Grade A laminar fl ow conditions).
µ
Aseptic filling under Class A,
laminar flow conditions
Door of
freeze drier
Class A or B background
Final product
container
0.22
µm filter
Final bulk
product
Sterile bulk
product
Freeze
drier
Figure 6.23
Final product fi lling. The fi nal bulk product (after addition of excipients and fi nal product QC
testing), is fi lter sterilized by passing through a 0.22
µ
m fi lter. The sterile product is aseptically fi lled into
(pre-sterile) fi nal product containers under grade A laminar fl ow conditions. Much of the fi lling operation uses
highly automated fi lling equipment. After fi lling, the product container is either sealed (by an automated
aseptic sealing system), or freeze-dried fi rst, followed by sealing








Search WWH ::

Custom Search