Biomedical Engineering Reference
In-Depth Information
+
+
+
+
+
+
+
+
Negatively charged
protein
Positively charged
ion-exchange bead
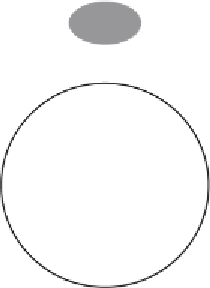
Figure 6.11
Principle of ion-exchange chromatography, in this case anion exchange chromatography. The
chromatographic beads exhibit an overall positive charge. Proteins displaying a nett negative charge at the
pH selected for the chromatography will bind to the beads due to electrostatic interactions
it leads to a concentration of the protein of interest. It is also one of the least expensive chromato-
graphic methods available. At physiological pH values most proteins exhibit a net negative charge.
Anion-exchange chromatography, therefore, is most commonly used.
6.6.3 Hydrophobic interaction chromatography
Of the 20 amino acids commonly found in proteins, eight are classifi ed as hydrophobic, due to
the non-polar nature of their side chains (R groups,
Figure
6.12). Most proteins are folded such
Table 6.3
Functional groups commonly attached to chromatographic beads in order to generate cation or
anion exchangers
Group name
Group structure
Exchanger type
!O!(CH
2
)
2
!NH
!(CH
2
!CH
3
)
2
Diethylaminoethyl (DEAE)
Anion
!CH
2
!NH
!(CH
3
)
3
Quaternary ammonium (Q)
Anion
Quaternary aminoethyl (QAE) !O!(CH
2
)
2
!N
(C
2
H
5
)
2
!CH
2
!CHOH!CH
3
Anion
!O!CH
2
!COO
Carboxymethyl (CM)
Cation
!CH
2
!SO
3
Methyl sulfonate (S)
Cation
!CH
2
!CH
2
!CH
2
SO
3
Sulfopropyl (SP)
Cation





Search WWH ::

Custom Search