Biomedical Engineering Reference
In-Depth Information
Endocrine
Paracrine
Cell recruitment
Fig. 3 The crosstalk between adipose tissue and tumors. Adipose tissue can interact with tumors
in different ways: endocrine (systemically circulating adipokines), and paracrine (adipokines
locally produced by WAT-derived cells infiltrating tumor microenvironment). Endocrine
adipokines may affect cancer progression through inducing changes in other endocrine organs,
such as brain, liver and pancreas. In addition, autocrine adipokine activity promotes remodeling
of WAT itself, leading to progressive pathogenesis in obesity
2.2 Adipose Cell Role in Tumor Immunomodulation
In obesity, WAT undergoes changes as a result of hypoxia, neovascularization,
recruitment of various cell populations, and ECM remodeling [
2
]. As adipocyte
hypertrophy reaches a threshold at which oxygenation becomes inadequate, adi-
pocyte cell death triggers recruitment of leukocytes. Downstream of hypoxia
pathway activation, secretion of such chemokines as monocyte chemoattractant
protein 1 (MCP1) drives monocytes into adipose tissue. The subsequent increased
production of inflammatory cytokines by infiltrating leukocytes, in combination
with the inability of adipose tissue to store the surplus free fatty acids, is a major
hallmark of adipose tissue dysfunction in obesity [
74
]. These obesity-associated
disturbances of WAT function are believed to play a crucial role in the develop-
ment of type-2 diabetes and other components of the metabolic syndrome,
including hypertension, hypertriglyceridemia, low HDL-cholesterol and hyper-
glycemia, as well as of cancer [
2
].
It is well recognized that inflammation and immune tolerance are key compo-
nents of cancer initiation and progression [
12
]. Various obesity-induced inflam-
matory signals released by WAT may be involved in carcinogenesis through their
effect on adjacent tissues, however evidence for this is insufficient at this point.
While the role of WAT in cancer initiation is unclear, a body of evidence points to
obesity-induced immunomodulation being a driver of cancer progression [
75
].













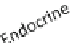


Search WWH ::

Custom Search