Biomedical Engineering Reference
In-Depth Information
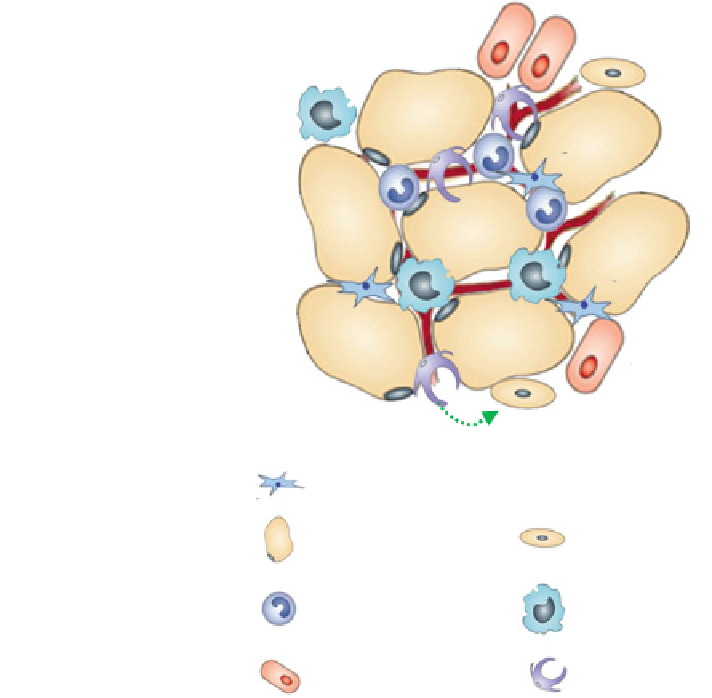
Fig. 1 Adipose tissue
heterogeneity and plasticity.
Adipose tissue consists of a
variety of cell populations:
adipocytes, pre-adipocytes,
adipocyte progenitor cells,
pericytes, endothelial cells,
and leukocytes (mainly
macrophages and other
monocytes). Green arrows
indicate reported
differentiation capacities of
individual cell populations
Adipose progenitor cell
Adipocyte
Pre-adipocyte
Monocyte
Macrophage
Endothelial cell
Pericyte
studies have tested the possibility that in obesity inflammation-initiated and
metabolic disorder-driven tissue remodeling could contribute to cancer progres-
sion. A building body of evidence indicates that the molecules secreted by WAT
(adipokines) nourish cancer cells, hence promoting tumor growth [
9
,
10
,
28
,
29
].
Moreover, recent data show that WAT-derived cells can traffic to the tumor site
where they have a potential for increased cancer-stimulating activity [
30
-
33
].
Cancer progression relies on the recruitment of stromal cells, a mixed population
of fibroblastoid cells of both mesenchymal and hematopoietic origins [
13
].
Collectively, these cancer-associated fibroblasts (CAF) [
34
] deposit extracellular
matrix (ECM) responsible for desmoplasia and contribute to the epithelial-
mesenchymal transition (EMT), a biological process in which epithelial cells lose
their epithelial characteristics and acquire a mesenchymal phenotype along with
increased migratory and invasive behavior [
12
,
35
]. In addition, they stimulate
vascularization and mute the anti-tumor immune response [
36
-
39
]. These effects
are executed by angiogenic, immunosuppressive, anti-apoptotic, and mitogenic













Search WWH ::

Custom Search