Chemistry Reference
In-Depth Information
more than 10
−6
M interfere; large amounts of tin(II) make it impossible to determine
phosphate and tin(IV) causes negative errors.
In later work, Motomizu
et al.
[68] point out that in the determination of phosphate at
µg L
−1
levels in waters the above method has certain disadvantages. First the absorbance
of the reagent blank becomes too large for the concentration effect achieved by the
solvent extraction to be of much use; for example when 20ml of sample solution and 5ml
of methyl isobutylketone were used, the absorbance of the reagent blank was 0.14.
Secondly, the shaking time needed was long and the colour of the extract faded gradually
if the shaking lasted more than 30min. In the
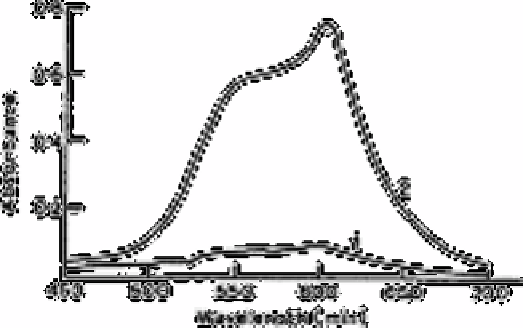
Fig. 15.1
Absorption spectra in the organic phase (procedure B) (1) reagent
blank measured against solvent; (2) 0.38µg P measured against
solvent
Source: Reproduced with permission from the Chemical Society of
Japan [66]
course of attempts to improve on this method, they observed that malachite green gave a
stable dark yellow species in 1.5M sulphuric acid (probably a protonated one), whereas
ethyl violet became colourless within 30min even in only 0.5M sulphuric acid.
Malachite green has several advantages over ethyl violet:
(1) the absorbance of the reagent blank is very small and 20-fold concentration of
phosphate by solvent extraction is possible;
(2) the reagent solution, which consists of malachite green, molybdate and 1.5M
sulphuric acid is stable at least for a month;
(3) the method is less troublesome and shaking for 5min is enough to complete the
extraction; and
(4) colour fading in the organic phase does not occur during shaking and standing.
The recovery of phosphorus was good, 99-103%. The relative standard deviation for
phosphorus was 0.6% for 21.0µg L
−1
in sea water (12 replicates) and 1.1% for 4.3µg L
−1
in potable water (10 replicates).

Search WWH ::

Custom Search