Chemistry Reference
In-Depth Information
at comparatively low concentrations (eg 50-150mg L
−1
) when these species are present
together in solution. The method uses sulphite ion as a mask to selectively and
quantitatively remove hypochlorite at pH 10.5. Thus hypochlorite is measured and
masked at the same time. At pH 10.5, sulphite does not react with chlorite and chlorate.
The remaining sulphite is quantitatively removed with triiodide ion. Following the
masking procedure, the sequential determination of chlorite and chlorate is carried out
either by iodometric titration at the appropriate pH or by ion chromatography. The results
from the direct potentiometric titration using sulphite are compared to those of an indirect
reference method.
Adam and Gordon [20] prepared high purity sodium hypochlorite by a previously
described procedure [21,22].
Gordon [23] has described a procedure for the determination of down to 0.1mg L
−1
of
chlorite and hypochlorite by a modified iodometric technique.
14.3.2
Trade effluents
14.3.2.1
Thiosulphates and polythionates
Makhija and Hitchen [24] determined these anions in mining effluents by an acidimetric
method. It involves sodium hydroxide titration of the acid liberated on addition of
mercuric chloride (which oxidises thiosulphate and polythionates to sulphuric acid), after
adjustment of the initial pH value to either 4.3 (using methyl orange) or 8.2 (using
phenolphthalein).
In this method a suitable portion of the solution containing a polythionate having 3, 4
or 5 sulphur atoms is acidified to either pH 4.3 or pH 8.9 with 0.005N sulphuric acid and
sodium hydroxide and mercuric chloride is added to release acid quantitatively. The
stoichiometric equation is:
A similar equation applies for the thiosulphate:
In both cases the acid generated is titrated with standard sodium hydroxide solution to
determine the sum of the concentration of the four sulphur anions:
14.3.2.2
Thiocyanates and cyanides
Atkinson
et al.
[25] have described a technique for titrating thiocyanate/ cyanide mixtures
in hydrometallurgical effluents. The mixtures are titrated with silver nitrate solution using
an automatic potentiometric titration with a silver/glass electrode pair. When thiocyanate
is to be determined, cyanide is masked with formaldehyde.

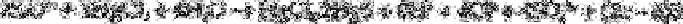

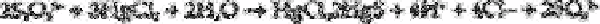
Search WWH ::

Custom Search