Chemistry Reference
In-Depth Information
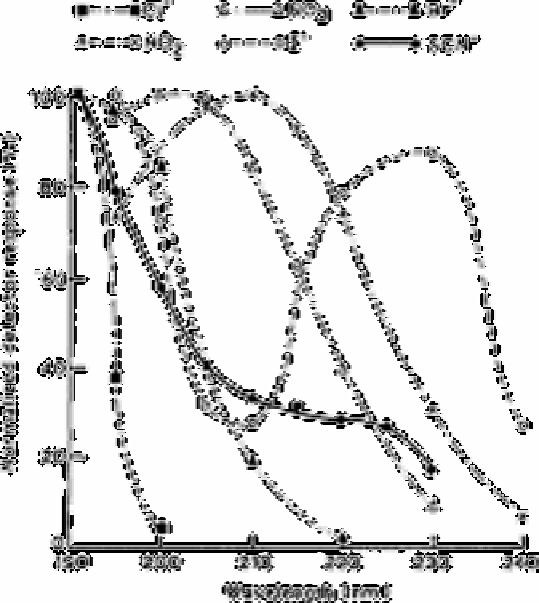
Fig. 13.1
Normalised detector response versus wavelength for Br
−
, Cl−, NO
2
−
,
NO
3
−
a
nd SCN showing the optimum absorption wavelengths
Source: Reproduced with permission from Groundwater Publishing
Co., Dublin, US [2]
method employing anion-exchange columns to ground water samples achieving detection
limits of 50µg L
−1
for these ions.
Thiocyanate, bromide, iodide, nitrate and nitrite have large enough extinction
coefficients at 194-215nm to make ultraviolet absorption detection useful for trace
analysis at these wavelengths. An eluant of phosphate buffer prepared with high
performance liquid chromatography grade water has no significant background
absorption to 190nm and could be used over the entire functional pH range of silica based
stationary phases. The choice of a suitable wavelength is generally limited only by the
purity of the eluant and the limits of the detector. Fig. 13.1 shows the area responses of a
10mg L
−1
bromide sample at several wavelengths. Wavelengths longer than 220nm are
not suitable for trace bromide analysis while those below 195nm show only marginal
increases in sensitivity. Thiocyanate demonstrates a similar wavelength absorptivity
relationship but its usable wavelengths limit extends to 235nm. The absorption spectrum
for the remaining anions are also shown in Fig. 13.1. Fig. 13.2 shows the results of the
analysis of a water sample containing chloride at
Search WWH ::

Custom Search