Chemistry Reference
In-Depth Information
Fig. 12.36
Chromatograms for sulphide and cyanide in deionised water and
potable water: (A) deionised water; (b) potable water sample
containing 260ng of sulphide and 850ng of cyanide; (c) potable water
sample as in (B) spiked with copper ions (2mg L
−1
) and the decrease
in the cyanide response can be seen
Source: Reproduced with permission from the American Chemical
Society [21]
Despite a higher sensitivity, the mercury film electrode has some disadvantages
compared to the dropping mercury electrode. Day to day reproducibility of the mercury
film and hence the analyte signal is harder to maintain than at the dropping mercury
electrode. If the film layer is too thin, bad tailing occurs (electrode saturates); the
electrode can also eventually become poisoned. The length of time it lasts will depend on
the nature and content of samples injected. Consequently more frequent calibration is
required than at a dropping mercury electrode.
Fig. 12.36 shows that the response in this water is not the same as in deionised water,
suggesting that only free sulphide and cyanide are being determined. This is not
surprising and is characteristic of most methods for determining sulphide or cyanide.
Since the drinking water was known to contain a significant amount of copper, some of
the cyanide may be bound to the metal. Deliberate addition of copper to the water
reduced the cyanide peak, supporting this hypothesis. The non-zero intercept for cyanide
calibration curves is believed to be a result of complexation by impurities.
Essentially identical results were obtained by using short drop times at the dropping
mercury electrode, dc mode (0V) or using the mercury coated platinum electrode, normal
pulse mode (pulse −0.80 to 0V, pulse delay=1s, pulse width=20ms.) Examination of
industrial effluents known to contain sulphide and cyanide demonstrate that the proposed
method works extremely well and that free cyanide rather than total is being determined.
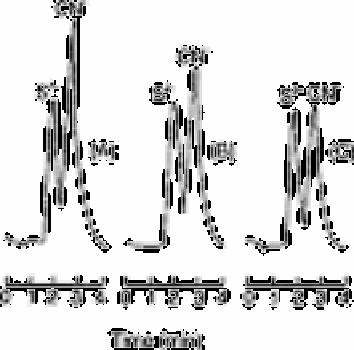
Search WWH ::

Custom Search