Chemistry Reference
In-Depth Information
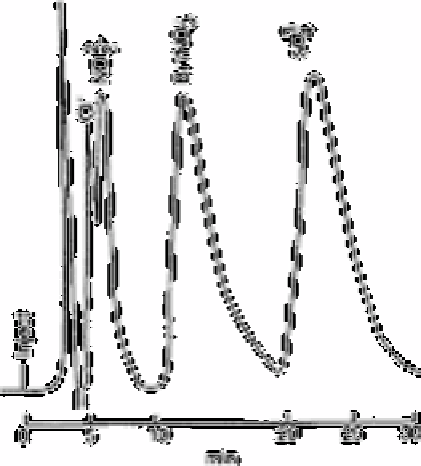
Fig. 12.35
Simultaneous elution of bromide and nitrate using standard eluent
Source: Reproduced with permission from Elsevier Science [71]
system using slightly weakened standard eluent (0.003mol L
−1
sodium
bicarbonate/0.0024mol L
−1
sodium carbonate) at 30% Q (2.3ml min
−1
). The auto sampler
was programmed to allow the ion chromatograph to remain in the inject position for the
remainder of each sample elution, after injection of the sample, so that a continual
flushing of the concentrator was accomplished prior to loading of the next sample.
When using conventional ion chromatographic separation techniques, it is possible that
other matrix anions also common to non saline waters may coelute with bromide. For
example, bromide and nitrate elute simultaneously using a standard anion separator
column (No. 30065), standard anion suppressor (No. 30366) and standard eluent
(0.003mol L
−1
sodium bicarbonate/0.0024mol L
−1
sodium carbonate at 30% flow
(7.67ml min
−1
). A representative chromatogram is shown in Fig. 12.35.
Separation of bromide from all other matrix anions was shown to be possible using a
trace anion separator (No. 30827), a standard anion suppressor, and slightly weakened
standard eluent, at 30% flow.
Chloride, nitrate and sulphate were the only ions found to be present in any significant
quantities in the representative raw waters analysed. To determine whether response for
bromide remained the same regardless of the concentration of these anions, recovery tests
were performed on bromide standard solutions of 80µg L
−1
spiked with varying
concentrations of chloride, nitrate and sulphate, using the concentrator found to have the
highest µ-equivalent capacity.
Search WWH ::

Custom Search