Chemistry Reference
In-Depth Information
for nitrate, 0.5mg L
−1
for sulphite and 0.1mg L
−1
for sulphate.
Little change was found in the concentration of anions during 3d storage when the
sample was kept at 25°C in a refrigerator.
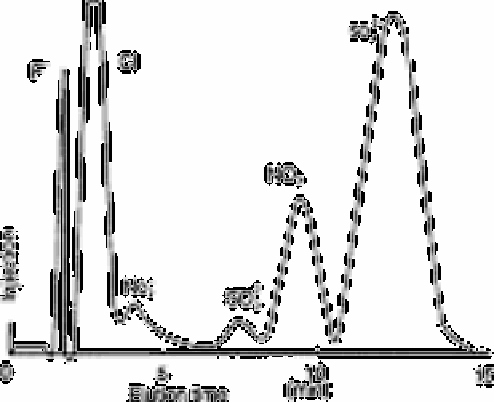
Fig. 12.28 shows an example of an ion chromatogram of rainwater. Using the
following conditions, Schwabe
et al.
[60] determined chloride, fluoride, nitrate,
phosphate and sulphate in 20min in rainwater and potable water in amounts down to
0.5mg L
−1
.
Type
Dionex D12
Sensitivity
1ks
Analytical column
Fast run anion separator
Suppressor column
Anion suppressor
Sample loop
100µL
115ml h
−1
Pump flow
Pressure
22 bar
Run time
11min per sample
0.0048mol L
−1
Na HCO
3
0.0023mol L
−1
Na
2
CO
3
Eluent
Fig. 12.29 shows the results for chloride, fluoride, nitrate and sulphate in Berlin
rainwater. The month of March shows significantly higher loads than the other months,
caused by high rainfall. There is also a high load of sulphate and nitrate for October and
November.
Fig. 12.28
Example of ion-chromatogram of rainwater
Source: Reproduced with permission from Elsevier Science [60]

Search WWH ::

Custom Search