Chemistry Reference
In-Depth Information
graphically in Fig. 12.20. In this equation, elution volume is used instead of retention
time; the two are related by the eluant flow rate.
(1)
V
r
is the retention volume, V
m
is the mobile phase volume (column void volume), A is
the analyte ion's charge, E is the eluant ion's charge, R is the gradient ramp steepness in
mM of eluant concentration per mL of eluant pumped, and log Const, is the Y intercept
of the log/log plot. This equation predicts that an increase in gradient steepness will
decrease the retention time of divalent ions more than monovalent ions. An advantage
monovalent eluants have over divalent (or other polyvalent) eluants is that a given change
in eluant concentration produces a greater change in analyte retention time. Compared to
a monovalent eluant during a gradient run, the concentration of a divalent eluant must be
changed by a much larger amount to elute ions of widely differing retention.
For ions of different charge, the elution order, and therefore the selectivity, can be
changed by adjusting the gradient. For example, if two ions with different charge co-
elute, an increase in gradient steepness will
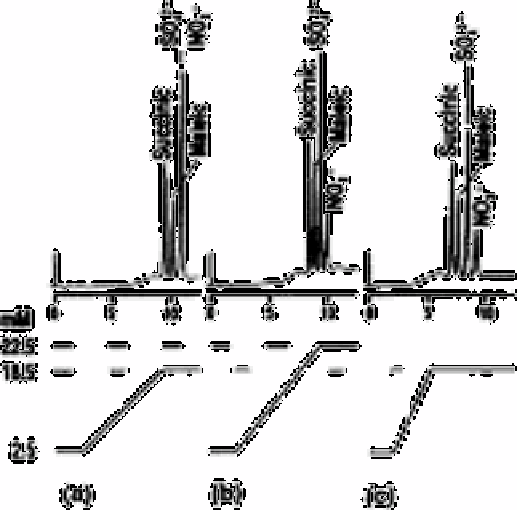
Fig. 12.21
Effect of gradient ramp on elution AS6
p
-cyanophenate eluant
Source: Reproduced with permission from Dionex Corporation [24]
separate the two, causing the ion of higher charge to elute first. In theory, one could
obtain the optimum conditions for a gradient elution from a plot such as that shown in

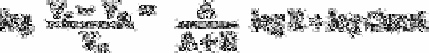
Search WWH ::

Custom Search