Chemistry Reference
In-Depth Information
by regenerating the column more frequently or by an increased use of standards with
samples.
Fig. 12.7
Chromatogram of sample 8 using the same conditions as for the
calibration in Fig. 12.6
Source: Reproduced with permission from Elsevier Science [10]
Typical chromatograms obtained from a sample of northern Ontario stream water are
shown in Fig. 12.7 (250mm column). No advantage is gained by using the 500mm
column rather than the 250mm column. The time for analysis is more than doubled and
peak heights have decreased. The increased separation of anion chromatographic peaks is
not required for this sample type.
The relationship between fluoride determination by ion selective electrode and ion
chromatography is good but a larger spread of values is evident with the 500mm column,
especially with samples containing above average amounts of fluoride. Values reported
by ion chromatography are usually higher than those obtained by the selective ion
technique. The minimum reported concentration for fluoride by ion chromatography is
3µg L
−1
and for selective ion electrodes 20µg L
−1
.
The detection limit of the 2-aminoperimidine method for sulphate is 5-10mg L
−1
.
Sulphate contents reported by both columns usually correlate well with one another. The
lower limit of detection for sulphate reported for the 250mm column is 0.05mg L
−1
and
for the 500ml column 0.05mg L
−1
two orders of magnitude below the limit reported for
the 2-aminoperimidine method.
A comparison between nitrate determination by ion chromatography and by the
colorimetric technique shows that there is a correlation between the two sets of results but
nitrate values obtained by the chromatographic method are consistently higher than those
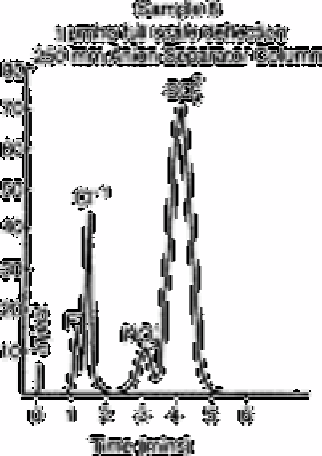
Search WWH ::

Custom Search