Chemistry Reference
In-Depth Information
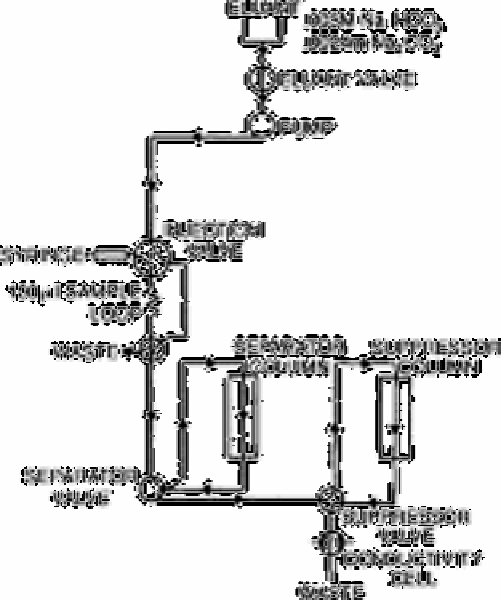
and recorder. Following the injection of a small volume (100µL) of sample, rapid
exchange and separation of the anions is accomplished with a specially prepared low
capacity resin (containing quaternary ammonium exchange sites existing as a thin film on
the surface to facilitate fast equilibrium).
The otherwise highly conducting background of the eluant (0.003mol L
−1
sodium
bicarbonate/0.0024mol L
−1
sodium carbonate) is virtually
Fig. 12.5
Ion chromatographic flow diagram. Eluant conditions used for anion
analysis
Source: Reproduced with permission from Elsevier Science [10]
eliminated by passage through the high capacity suppressor column whereupon carbonic
acid is formed together with the acid forms of the anions of interest. The conductivity
meter used as a detector then only sees a small residual background due to the weakly
dissociated carbonic acid and the conductivity of the acids of interest separated in time.
Additional to this basic instrumentation, there are four reservoirs (2 eluant, 1 regenerant,
1 water), a sample injection valve with a 100µL loop, two Milton Roy fluid pumps with
adjustable flow rates and an automatic timer for controlling the regeneration cycle of the
cation exchange resin.
Approximately 1ml of untreated sample was injected into the entrance port by means
of a hypodermic syringe; 100µL of sample was used to fill the sample loop, the
Search WWH ::

Custom Search