Chemistry Reference
In-Depth Information
plant, where chloride levels may be as low as a few micrograms per litre.
11.2.1.1
Titration method
A titrimetric method for the determination of milligram per litre quantities of chloride
(and bromide) in boiler feed water involves titration of the sample at pH 1 to 4 with 0.1N
mercuric nitrate to the tetra(ethyl di-(1-sodiotetrazol-5-ylazo)acetate yellow to red end
point [7]. A 300-fold excess of sulphate, nitrate, carbonate or acetate can be tolerated, but
cobalt, fluoride, iodide or nickel interfere.
Samples can be acidified with perchloric acid and ferric nitrate solution added
followed by ethanolic [8] or methanolic [10] thiocyanate.
The extinction of the mercuric thiocyanate complex is measured at 460nm [8] or 463nm
[10]. Chloride can be determined down to 1µg L
−1
by these procedures. Hydrogen
peroxide or hydrazine used as boiler water treatment chemicals do not interfere.
Interference arises from the presence of comparatively large amounts of cupric sulphate,
potassium dichromate, lithium hydroxide or ammonium fluoride. The coefficient of
variation at the 50µg L
−1
level was 12%.
11.2.1.2
Spectrophotometric method
Two Spectrophotometric procedures involving the formation of the red coloured ferric
thiocyanate complex formed between chloride ion and ethanolic mercuric thiocyanate in
the presence of ferric ions have been described for the determination of chloride in boiler
feed water.
In these methods the sample is acidified with perchloric acid or nitric acid and ferric
nitrate solution is added followed by ethanolic [8] or methanolic [11] thiocyanate. The
extinction of the mercuric thiocyanate complex is measured at 460nm or 463nm [11].
Down to 1µg L
−1
chloride can be determined by these procedures. Hydrogen peroxide
and hydrazine used as boiler water treatment chemicals did not interfere. Interference
arises from the presence of comparatively large amounts of cupric sulphate, potassium
dichromate, lithium hydroxide or ammonium fluoride. The coefficient of variation at the
50µg L
−1
level was 12%.
11.2.1.3
Ion selective electrodes
Silver-silver chloride electrodes [11-14] and solid state mercurous chloride electrodes

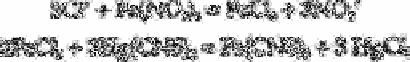

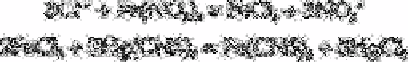
Search WWH ::

Custom Search