Chemistry Reference
In-Depth Information
and thiocyanate more sensitive and reproducible by adopting the head space technique.
This technique lends itself to automation.
Nota
et al.
[25] have also described a gas chromatographic head space method based
on different principles for the determination of 0.01-1 00mg L
−1
of cyanide and
thiocyanate in coke oven waters and waste effluents. This method involves firstly
transforming the cyanides, or the thiocyanates, into hydrogen cyanide by acidification,
then removing hydrogen cyanide from the aqueous sample by the head space technique
and finally separation of hydrogen cyanide by gas solid chromatography and selective
detection with a nitrogen phosphorus detector. This procedure for the determination of
cyanide is based on three stages: first, transformation into hydrogen cyanide by
acidification; second, removal of hydrogen cyanide by the head space technique, and
third, gas chromatographic separation of hydrogen cyanide and selective detection with a
nitrogen phosphorus detector. A similar procedure is adopted for the determination of
thiocyanate, the only difference being the quantitative transformation of thiocyanate into
hydrogen cyanide according to the reactions:
(where If cyanide is present prior to the
oxidation step, it must be transformed into unreactive cyanohydrin by an excess of
formaldehyde or removed by boiling the solution previously acidified to pH 2.
The presence of iron(II), iron(III) and copper(II) in the sample decreases the response
for hydrogen cyanide. The greatest effect is caused by copper (II). Reducing agents do
not interfere in the analysis of cyanide but oxidising substances have to be reduced prior
to heating of the sample. Oxidants and reducing agents do not interfere in the
determination of thiocyanate.
10.4.10
Ion chromatography
The application of this technique is discussed under multianion analysis in section 12.8.2.
10.4.11
Miscellaneous
Gregorowicz and Gorka [26] have reviewed methods for the determination of cyanide in
industrial effluents.
Cyanide and cyanate in waste water have also been separated by paper
chromatography [27]. Separation is achieved by use of isopropyl alcohol:ethanol:water
(9:4.3) as solvent with Filtrak FN 8 paper. After drying, the paper is sprayed with
bromocresol green solution. On a green background the ions appear as clear blue spots
(RF values-cyanide 0.04, cyanate 0.29). The spots may also be located by treating the
paper with silver nitrate solution and exposing it to hydrogen sulphide.

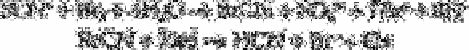
Search WWH ::

Custom Search