Chemistry Reference
In-Depth Information
cyanides, or the thiocyanates,
Table 8.13
CN
−
determination in some typical samples of coke-oven water (A-E) and
coke-oven water effluent into the sea (F-J)
Sample
mg L
−1
CN
−
added
CN
−
recovered gas
chromatographic method
Standard
methods
Gas
chromatographic
method
Standard
methods
A
39.0
44.0
20.0
18.6
20.2
B
50.0
55.0
20.0
17.5
20.3
C
62.0
70.0
25.0
23.0
25.1
D
47.0
53.0
5.0
4.6
4.91
E
46.0
52.0
5.0
4.5
4.93
F
0.27
0.295
0.150
0.134
0.147
G
0.27
0.307
0.150
0.137
0.154
H
0.31
0.353
0.175
0.150
0.178
I
0.25
0.263
0.050
n.d.
0.047
J
0.37
0.414
0.50
n.d.
0.048
To each sample a known amount of KCN was added to check the recovery. Concentrations are
expressed in ppm
Source: Reproduced with permission from Elsevier Science [43]
into hydrogen cyanide by acidification with 85% phosphoric acid, then removing
hydrogen cyanide from the aqueous sample by the headspace technique, and finally
separating hydrogen cyanide by gas solid chromatography and selective detection with a
nitrogen phosphorus detector. Any oxidising agents present in the sample must be
reduced with sodium sulphite prior to heating the sample.
A similar procedure is adopted for the determination of thiocyanate, the only difference
being the quantitative transformation of thiocyanate into hydrogen cyanide according to
the reactions:
(where Red=SO
2
or I
′
and Ox=SO
4
2−
or I
3
−
). If cyanide is present prior to the oxidation
step, it must be transformed into unreactive cyanohydrin by an excess of formaldehyde or
removed by boiling the solution previously acidified to pH 2.
The headspace conditions were as follows: needle temperature, 100°C; flushing of


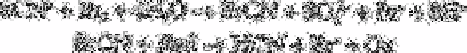



Search WWH ::

Custom Search