Chemistry Reference
In-Depth Information
determination of cyanide [36-39]. Sulphide ion is an interferent, a cadmium nitrate
treatment has been found to remove sulphide without influencing the response of the
silver iodide-silver sulphide electrode. If both the precipitate and solution from this
treatment are colourless (sulphide absent) filtration of the mixture before the
potentiometric measurement is unnecessary. Since the electrode response of the electrode
is pH dependent, standards and samples must be brought to the same pH. It is noteworthy
that the silver/silver sulphide electrode (eg Orion 94-16) is more sensitive to cyanide
than the silver iodide-silver sulphide one [38,40], but the response is affected by
cadmium ion.
Fraut
et al.
[40] have described an electrode indicator technique for the determination
of low levels of free cyanide in waste waters. Concentrations of cyanide down to 30µg
L
−1
are determined with the use of the Ag
+
selective electrode and 10µM Ag(CN)
2
−
as
indicator solution. The pH of the sample is adjusted to between 11 and 12 and an addition
procedure is used, the concentration of cyanide in the sample being obtained by use of a
Gran plot; samples containing more than 260mg L
−1
of cyanide must be diluted initially.
Copper and nickel are masked with EDTA and any sulphide removed by addition of lead
ions in slight excess. Ammonia does not interfere even in a 1000-fold excess.
8.8.7
Gas chromatography
Funazo
et al.
[41] have described a method for the determination of cyanide in water in
which the cyanide ion is converted into benzonitrile by reaction with aniline, sodium
nitrite and cupric sulphate. The benzonitrile is extracted into chloroform and determined
by gas chromatography with a flame ionisation detector. The detection limit for
potassium cyanide is 3mg L
−1
. Lead, zinc and sulphide ion interfere at 100mg L
−1
but not
at 10mg L
−1
.
More recently, Funazo
et al.
[42] have described a more sensitive gas chromatographic
procedure capable of determining down to 3µg L
−1
total cyanide in waste waters. The
method is based on the derivatisation of cyanide to benzonitrile, which is extracted with
benzene and determined

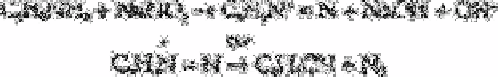
Search WWH ::

Custom Search