Chemistry Reference
In-Depth Information
7.18.3
Continuous flow analysis
Thompson and Blankley [49] have described an automatic ultraviolet continuous flow
determination of nitrate in raw and potable water. The method has a linear calibration
range of 0-30µg L
−1
nitrate N and a detection limit of 0.01µg L
−1
.
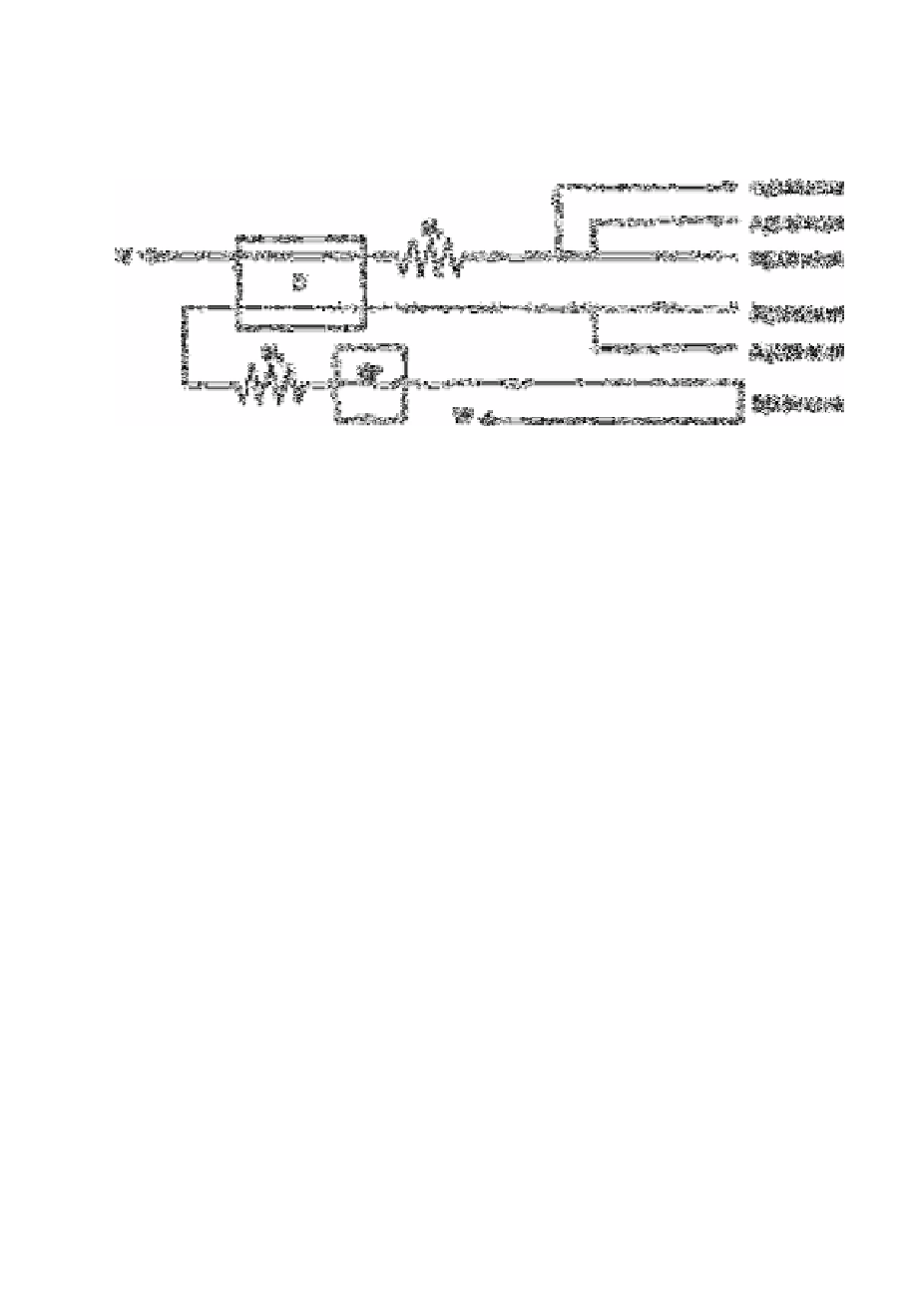
Fig. 7.10
Diagram of the continuous flow manifold Air (A
1
) is used to segment
the flow of sample (S) that is mixed with reagent RI in a four-turn
20mm diameter mixing coil M, and pumped through one half of the
150mm diameter (D) to waste (W). Reagent R
2
is segmented with air
(A
2
) pumped through the other half of the dialyser and mixed in coil
M
2
with dialysed sample. Mixing coils M, and M
2
are identical.
Nitrate absorbance at 210nm is measured by spectrophotometer SP
where part of the flow is pulled through a 1 0mm silica flow cell by
pump tube B
Reagent
R
1
Dissolve 100g of sulphamic acid in about 500ml of water,
carefully add 5.0mol of sulphuric acid (98 wtT) followed by 4.00g of
hydroxylamine sulphate and 3 drops of a 30% w/v Brij 35 solution
(BDH Chemicals). Dilute to 1L with water. Prepare freshly ever 2
weeks. The hydroxy-amine sulphate was added to reduce any
chromium(VI), iron(III), chromium(III) and iron(II) respectively. The
lower oxidation states of these elements do not significant absorb at
210nm at the maximum concentrations likely to be encountered.
Reagent R
2
Dissolve 10g of anhydrous sodium sulphate in approximately
500ml of water, add 3 drops of 30% w/v Brij 35 solution and dilute to
1L
Source: Reproduced with permission from the Royal Society of
Chemistry [49]
The incorporation of a dialysis membrane minimised the effect of humic acid type
substances that did not freely diffuse through the membrane, and also avoided the
necessity to filter water samples prior to measurement.
The method requires no sample pre-treatment or dilution. It utilises a conventional
dialysis membrane to minimise interference effects from suspended, colloidal and
Search WWH ::

Custom Search