Chemistry Reference
In-Depth Information
a cation-exchange resin in the K
+
form (TSK SCX Tµm;
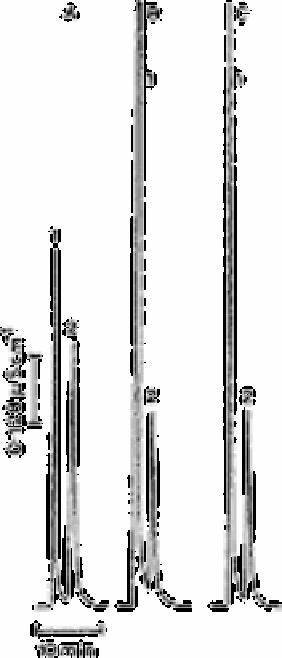
Fig. 7.5
Ion exclusion chromatograms of HCO
3
−
in some tap waters: (A) raw
tap water of City of Ames, IA, after 10-fold dilution (0.653mM×10);
(B) potable water after softening treatment (0.466mM); (C) potable
water (La Salle, 1L) after 20-fold dilution (0.470mM×10). Peak 1 is
strong acid anions and peak 2 is HCO
3
−
Source: Reproduced with permission from the American Chemical
Society [8]
TSK IC-Cation for cation chromatographic use, 10µm silicon based material with low
cation-exchange capacity).
The second enhancement column was constructed of plastic (4.6× 50mm) and packed
with an anion-exchange resin in the OH+ form TSK SAX (5µm). The precolumn was
constructed of plastic (7.5×100mm) and packed with an anion exchange resin in the OH+
form (TSK SAX, 5µm).
All samples were filtered with a 0.45µm polytetrafluoroethylene (PTFE) membrane
filter before injection into the column.
Tanaka and Fritz [8] applied this procedure to the determination of bicarbonate or
Search WWH ::

Custom Search