Chemistry Reference
In-Depth Information
three procedures were compared with those based on the thorin-barium reaction in a
continuous flow analyser. The chemical system employed in the first three methods
mentioned above involves basically a complex of barium ions with the indicator
dimethylsulphonazo(III). Sulphate ions from the sample react with barium and set free
the uncomplexed dimethylsulphonazo(III). The absorption spectra of complexed and free
dimethylsulphonazo(III) are different.
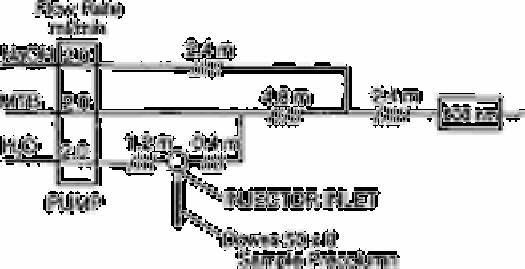
Fig. 5.1
Components of flow injection system
Source: Reproduced with permission from the American Chemical
Society [39]
There is no significant difference in the results between the methods.
Reijnders
et al.
[37] concluded that segmented continuous flow analysis and flow
through titrimetry are suitable methods for the determination of sulphate in real samples.
Results of (segmented) flow injection analysis and flow through titrimetry are less
affected by interfering ions. The dimethylsulphonazo(III) chemical system is superior to
the system with thorin.
Madsen and Murphy [39] adapted the automated methylthymol blue method for the
determination of sulphate in rainwater to flow injection analysis processing. The
calibration curve is linear up to 6mg L
−1
, sensitivity is approximately 0.1mg L
−1
and
sampling rate is 20h
−1
. Average precision and accuracy are 4.1% and 97% respectively.
The flow injection system used is shown in Fig. 5.1. The sodium hydroxide reagent
comprised 0.036mol L
−1
sodium hydroxide in 40 vol% ethanol water. The methylthymol
blue reagent which gave the highest sensitivity was prepared as follows: 0.116g
methylthymol blue was dissolved in a solution which contained 6.0ml of 1.0mol L
−1
hydrochloric acid, 80ml of deionised water and 14.0ml or 1.526gL
−1
barium chloride
dihydrate solution. Final dilution with 95% ethanol was to 1.01. Fresh reagent must be
prepared daily.
The indirect determination of sulphate based on competitive reaction of sulphate and
methylthymol blue with barium in solution can be accomplished by measurement of
absorbance due to either uncomplexed methylthymol blue or the methylthymol blue
barium complex. Absorbance at 460nm due to uncomplexed methylthymol blue will
increase while absorbance due to the methylthymol blue barium complex will decrease as
sulphate concentration increases.
Search WWH ::

Custom Search