Chemistry Reference
In-Depth Information
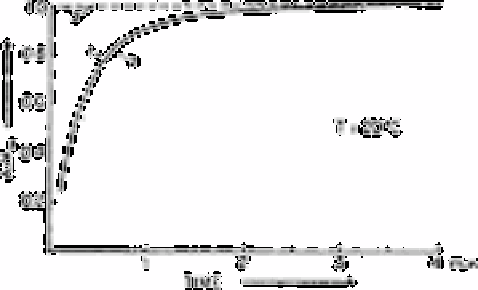
(line 1). The rates of formation of molybdenum blue from β-molybdosilic acid using
ascorbic acid (line 2) and stannous chloride (line 3) as the reductants are also shown. Line
1 was obtained from the absorbance measured at 390nm, while the absorbance at 810nm
was recorded to obtain lines 2 and 3. All lines have been normalised to the absorbance A
∞
measured after completion of the reaction.
The formation of molybdosilic acid is the rate limiting step in flow injection analysis
when stannous chloride is used as a reductant. The
Fig. 3.18
Rate of formation of β-molybdosilic acid (line 1) and of molybdenum
blue using ascorbic acid (line 2) and stannous chloride (line 2) as the
reductants
Source: Reproduced with permission from the American Chemical
Society [197]
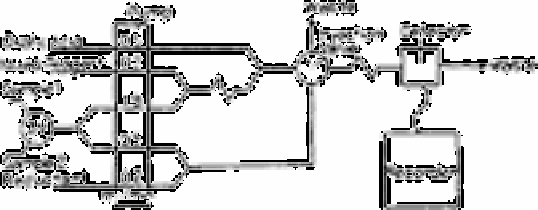
Fig. 3.19
Block diagram for the determination of silicate by flow injection
analysis
Source: Reproduced with permission from the American Chemical
Society [197]
reduction of the molybdosilic acid by ascorbic acid is slower than with stannous chloride.
The reduction step will also limit the extent of the reaction when ascorbic acid is used.
A block diagram of the manifold used by Thompson
et al.
[197] is shown in Fig. 3.19.
A four-channel peristaltic pump (Buchler, Polystaltic) was used to propel five solutions
by placing two pump tubes in one channel. Vinyl pump tubing with i.d. of 1.52mm for
Search WWH ::

Custom Search