Chemistry Reference
In-Depth Information
inorganic salts
NO
3
N by
polarography
1.170
*Addition of exact amount of silver sulphate to precipitate chloride with no excess silver
†Addition of sulphamic acid (final concentration, in sample approximately 20mg L
−1
)
‡Treatment with Amberlite XAD-2 resin
§Addition of saturated silver sulphate (1-10ml sample). Not recalibrated
¥Addition of 2M acetic acid to sample (0.09ml per 30ml sample). Not recalibrated
¶Calibrate for presence of 3ml saturated silver sulphate and 0.09ml 2M acetic acid in 30ml
sample
**Daz—a commercially available household detergent
Source: Reproduced with permission from Elsevier Science [127]
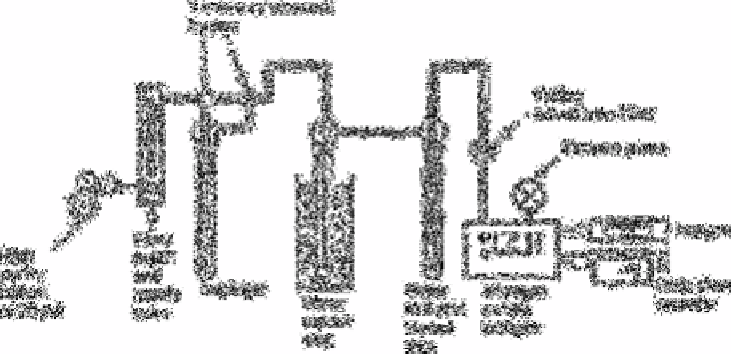
Fig. 3.7
Schematic diagram of apparatus
Source: Reproduced with permission from Elsevier Science [128]
return of the nitrogen dioxide to the ground state is accompanied by release of a photon
which is detected by a photomultiplier. The integrated output of the photomultiplier over
the time that the nitric oxide is purged from the sample is proportional to the nitrate or
nitrite content of the sample.
The apparatus is shown schematically in Fig. 3.7. Helium is supplied to the system at a
pressure of 20psi (138kpa) and the flow is regulated by means of a precision needle valve
and flowmeter. The flow is adjusted to provide a small positive pressure at the input to
the nitrogen oxides analyser. The helium flow is directed through an impinger consisting
of a coarse porosity sintered glass frit (20-50µm) in a 20×3.5cm diameter glass sample
tube fitted with a 24/40 ground-glass joint. The sample tube can be bypassed from the
helium flow by means of a pair of three-way stopcocks to allow changing of samples.
Polyethylene 0.6cm od tubing and nylon Swagelock connectors are used at this point in

Search WWH ::

Custom Search