Chemistry Reference
In-Depth Information
and the spectrophotometric method after conversion of iodate to I
3
−
in acidic solution
[78,85,94]. Total inorganic iodine was determined after converting iodide to iodate by
using chemical or photochemical means [78,83,85,86,88,89,92,94,95]. However, as the
detection limit of iodide by the difference technique is not very good (for example, 1.3-
2.5µg L
−1
for differential pulse polarography [83,89]), it is desirable to detect iodide
directly.
To date, a few methods have been proposed for direct determination of trace iodide in
seawater. The first involved the use of neutron activation analysis [87], where iodide in
seawater was concentrated by a strongly basic anion-exchange column, eluted by sodium
nitrate, and precipitated as palladium iodide. The second involved the use of automated
electrochemical procedures [83,91]; iodide was electrochemically oxidised to iodine and
was concentrated on a carbon wool electrode. After removal of interference ions, the
iodine was eluted with ascorbic acid and was determined by a polished Ag
3
SI electrode.
The third method involved the use of cathodic stripping square wave voltammetry [93].
Iodide reacts with mercury in a one-electron process, and the sensitivity is increased
remarkably by the addition of Triton X. The three methods have detection limits of 0.7
(250mL of seawater sample), 0.1 (50mL), and 0.02µg L
−1
(10mL), respectively, and
could be applied to almost all the samples. However, neutron activation analysis is not
generally employed. The second method uses an automated system but is a special
apparatus just for determination of iodide. The first and third methods are time-
consuming.
Recently, attention has been paid to ion chromatography as a simple method for
determining trace iodide in seawater [96,98-102]. Two factors are essential in order to
attain good sensitivity:
(1) separation of iodide from an excess of anions in seawater and
(2) highly sensitive detection of iodide.
Iodide was determined by an iodide-selective electrode (Ag
2
S/AgI) after other anions
were separated by sodium nitrate eluent [98]. However, the electrode that was stabilised
by 0.5µM iodide responded to chloride ion in seawater, and the detection limit of iodide
was ~2µg L
−1
Ito [103] (see section 3.18.4) showed that 0.1M sodium chloride eluent
was effective for the separation of iodide in seawater, due to faster elution of an excess of
anions in seawater and slow elution of iodide with hydrophobicity on conventional low-
capacity anion-exchange columns.
3.18.1
Titration method
Iodine in seawater can be determined by the procedure described below which is capable
of determining down to 0.1mg L
−1
iodide [104]. In this method the sample is strongly
acidified with hydrochloric acid titrated with 0.00125M potassium iodate solution which
converts iodide via iodine into iodine monochloride:
The end-point, which occurs with the complete conversion of iodide to iodine


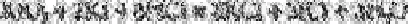
Search WWH ::

Custom Search