Chemistry Reference
In-Depth Information
merged with an acid stream. Sulphur dioxide is formed in the resulting stream.
This stream is diffused through a gas permeable membrane into a reagent stream
containing formaldehyde which is then merged with a stream of
p
-rosaniline to form a
red-purple complex which is measured at 560nm.
The application of this technique is also discussed under multianion analysis in section
14.4.1.1.
2.95.7
Ion selective electrodes
Sulphite (and thiosulphate) can be titrated potentiometrically with mercuric ions using a
sulphide ion selective electrode as indicator electrode in 0.1N sodium hydroxide medium.
Sulphide and polysulphides interfere in this procedure [853].
2.95.8
Enzymatic assay
Guilbault
et al.
[229] have shown that sulphite can be determined by its inhibitory action
over the enzymatic activity of the hydraluronidase enzymatic system.
2.95.9
Ion chromatography
Ion chromatography has been applied to the determination of sulphite and arsenate [854],
and sulphite and selenite [855] in non saline waters.
Grados
et al.
[856] have described an ion chromatographic method for the
determination of sulphite in non saline waters in the presence of other anions. The
application of this technique is also discussed under multianion analysis in section 12.2.5.
2.95.10
High performance liquid chromatography
A sensitive method for sulphite is based on high performance liquid chromatography
followed by detection using inductively coupled plasma atomic emission spectroscopy
[857].
2.95.11
Ion exclusion chromatography
The application of this technique is discussed under multianion analysis in section
13.2.1.1.
2.95.12
Micelle chromatography
The application of this technique is discussed under multianion analysis in section
13.5.1.1.

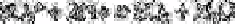
Search WWH ::

Custom Search