Chemistry Reference
In-Depth Information
Time/min
Re-extraction method
Methylene blue method
S
2−
found/mg L
−1
Decoloration
value
S
2
−
found/mg L
−1
Absorbance
Sample
1
Sample
2
Sample
1
Sample
2
Sample
1
Sample
2
Sample
1
Sample
2
10
0.830
0.602
1.12
0.81
0.562
0.383
1.20
0.81
30
0.828
0.600
1.12
0.81
0.568
0.381
1.20
0.81
60
0.808
0.593
1.10
0.80
0.550
0.385
1.16
0.82
90
0.781
0.575
1.05
0.78
0.525
0.375
1.10
0.80
120
0.753
0.542
1.02
0.73
0.508
0.347
1.07
0.73
Source: Reproduced with permission from the Royal Society of Chemistry [815]
The oxidation of thiamine to thiochrome by oxidants such as permanganate is subject to
interference by sulphides in the medium [817] because of the involvement of two
competing reactions with permanganate, that of oxidation of thiamine and that of
sulphide. A quenching method to determine sulphide has been proposed, with excellent
reproducibility, but diverse ions can interfere by reacting with sulphide, thiamine or
permanganate.
Bark and Rixon [818] described the determination of trace amounts of sulphide with
mercury(II)-2,2
′
-pyridylbenzimidazole. This chelate, with the probable structure (4), is
non-fluorescent and the free reagent is very
(4)
fluorescent. If the sulphide ion is added to the system containing the mercury(II)
complex, mercury(II) sulphide is formed and an equivalent amount of the fluorescent
organic ligand is released. Thus the fluorescence intensity of the system is increased.
When a large excess of acetate buffer is added to the system, some of the chloride may be
replaced by acetate and the authors suggested the following reactions for release of the
ligand:
where L represents ligand and Ac represents CH
3
COO
−
.
Sulphide can be determined by its inhibitory action over the enzymatic activity of the


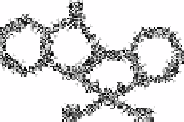

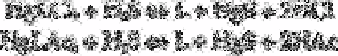


Search WWH ::

Custom Search