Chemistry Reference
In-Depth Information
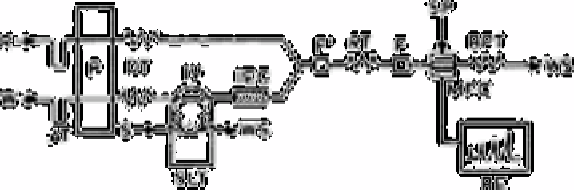
Fig. 2.52
Schematic flow diagram. R, reagent solution; W, water saturated with
BaSO
4
; AT, air trap; P, double plunger micro pump; DT, damping
coil; IV, injection valve; S, sample injection; SLT, sample injector
(1.0mm tubing 130µL); WS, waste; IEC, cation exchange resin
column; F, line filter; RT, reaction coil; SP, spectrophotometer;
MFC, micro flow cell; BPT, back pressure soil; RE, recorder
Source: Reproduced with permission from Springer Verlag [773]
sodium, 80mg L
−1
of potassium and 100mg L
−1
(maximum concentrations tested) of
chloride, nitrate, phosphate, hydrogen carbonate and silicate. These ions therefore did not
interfere with sulphate determination at levels which are normally present in non saline
waters. The lifetime of the cation exchange resin column was found to be at least two
months when regularly used.
Calibration graphs were linear over the sulphate range 0-14mg L
−1
and slightly curved
over the range 14-32mg L
−1
. The relative standard deviations of 20 analyses of solutions
containing 6 and 10mg L
−1
of sulphate were 0.94 and 1.2% respectively. The detection
limit was found to be about 0.2mg L
−1
of sulphate ion. The sampling rate was 20-30
samples per h.
Sonne and Dasgupta [774] carried out a simultaneous photometric flow injection
determination of sulphate, sulphide, polysulphide, sulphite and thiosulphate.
Eremina
et al.
[775] used flow injection spectrophotometry to determine sulphate at
concentrations of 0.5-20mg L
−1
; the sulphate in non saline waters masked the reaction of
barium with Orthanilic K.
Van Staden [776] presented a procedure for the on-line automated monitoring of
sulphate in effluent streams by reversed flow injection. The sampling capacity was 60
sulphate determinations per h, with alternate sampling of the barium chloride reagent and
the buffer EDTA solution. Results compared favourably with those of normal flow
injection and a standard automated segmented method.
Tang and Huang [777] have described a method for determining sulphate based on a
potentiometry-based linear calibration graph. These workers point out that although
various potentiometric procedures have been developed, a non-linear or semi-linear plot
is usually obtained and used as the calibration graph, or the equilibrium constant for the
reaction of sulphate with lead has to be known or determined in order to linearise the
calibration graph [766-768]. This introduces either unpredictable uncertainties or tedious
procedures. In order to overcome these drawbacks, the derived equations based on the
behaviour of the lead ion electrode and the interaction between a sulphate-containing
Search WWH ::

Custom Search