Chemistry Reference
In-Depth Information
Reaction of sodium iodate in the percolate with sodium iodide in hydrochloric acid
medium leads to the production of an amount of iodine equivalent to the sulphate content
of the sample. Iodine is titrated with 0.05N sodium thiosulphate to the starch end-point.
In the determination of 250mg L
−1
sulphate in non saline water by this method, 250mg
L
−1
of sodium, potassium, calcium, chloride or nitrate did not interfere. Phosphate
(100mg L
−1
) or bicarbonate (200mg L
−1
) caused an error of +10%.
Some of the chromophoric reagents that have been used to estimate sulphate are
reviewed in Table 2.47.
Reijnders
et al.
[722] have described the following batchwise photometric method for
the determination of sulphate in water. In this method metal ions are removed from the
sample by ion exchange and the sulphate reacts with the barium complex of
dimethylsulphonazo(III) in an ethanolic medium. The optical absorbance of the complex
indicates the sulphate concentration.
The results of determinations of pure sulphate solutions, containing 1-60µmol L
−1
sulphate are presented in Table 2.48. Table 2.49 shows the effect of different amounts of
interfering substances on the results of sulphate standards. The lower limit of
determination, defined as the concentration at which the coefficient of variance is 24%,
was 2.6µmol L
−1
Table 2.49
Effect of different amounts of interfering substances on the results of sulphate
standards
Sulphate given
µmol L
−1
Interference
Sulphate found
µmol L
−1
0
1
2.6
0
2
4
0
3
8
50
1
51
50
2
53
50
3
53
100
1
100
100
2
100
100
3
102
Key to interference:
1 No interferences
2 Sulphate and interference as specified in 3 in 1:1 dilution
3 Sulphate and 0.01mol L
−1
NaCl, 0.00 15mol KCl, 0.01mol L
−1
L
−1
NaHCO
3
, 0.05mol L
−1
I Fe
(NO
3
)
3
, 0.01mol L
−1
Ca(NO
3
)
2

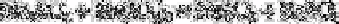



Search WWH ::

Custom Search