Chemistry Reference
In-Depth Information
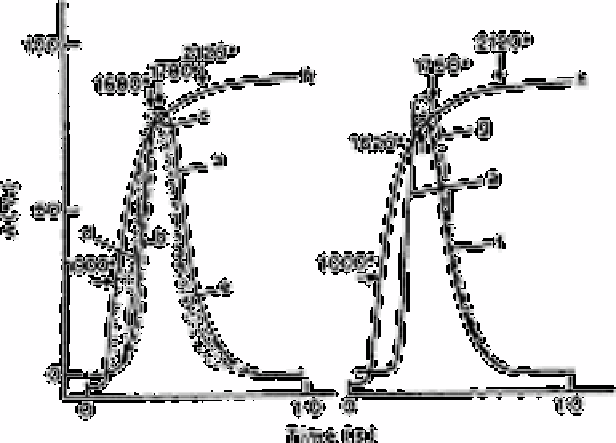
Fig.2.46
Memoriscope traces for effect of diverse elements; (a) 0.63ng Se(IV);
(b) 0.63ng Se(IV) and 63ng Pb; (c) 0.63ng Se(IV), 63ng Pb and 63ng
Cu; (d) 0.63ng Se(IV) and 63ng As: (e) 0.63ng Se(IV), 63ngAs and
63ng Cu; (f) 0.63ng Se(IV) and 63ng Te; (g) 0.63ng Se(IV), 63ng Te
and 63mg Cu; (h) temperature increase. Gas flow rate 500ml Ar
min
−1
Source: Reproduced with permission from American Chemical Society
[674]
2.90
Selenate and selenite
2.90.1
Atomic absorption spectrometry
Ohta and Suzuki [674] using a molybdenum micro-tube atomiser have developed an
atomisation technique for the determination of selenium in water samples. The highest
absorption peak was achieved in pure oxygen, as was the lowest atomisation temperature,
the analytical wavelength was 196.00nm. Extraction of selenium with
diethyldithiocarbamate eliminated matrix interference and copper addition suppressed
interference from co-extracted elements. No selenium(VI) was extractable from citrate
buffered solutions containing EDTA, so that use of these reagents allowed separate
determination of selenium(IV) and selenium(VI). Recoveries of 90-106% were achieved
in analysis of water samples containing 30-130µg L
−1
selenium.
Most cations and anions have interferences on atomisation of selenium. The effects of
100-fold amounts of lead, arsenic and tellurium on atomisation of selenium are shown in
Fig. 2.46. Samples were checked for background effects and reagent blank. Bismuth and
antimony also depressed the selenium absorption.
Search WWH ::

Custom Search