Chemistry Reference
In-Depth Information
nitrite and nitrate. These ions are reduced to nitric oxide with hydroquinoline and then
nitric oxide purged from the sample and oxidised at a membrane covered anodically
polarised platinum electrode.
2.64.9
Column coupling capillary isotachophoresis
This technique offers many similar advantages to ion chromatography in the
determination of anions, namely multiple ion analysis, little or no sample preparation,
speed, sensitivity and automation. Very similar advantages are offered by capillary
isotachophoresis. Zelinski
et al.
[190] applied the technique to the determination of
nitrite, nitrate, fluoride, chloride, sulphate and phosphate in river water.
The application of this technique is also discussed under multianion analysis in section
14.6.1.1.
2.64.10
Ion selective electrodes
Zhou [573] has described the use of an ammonia sensor probe followed by reduction for
the determination of nitrate plus nitrite in non saline waters.
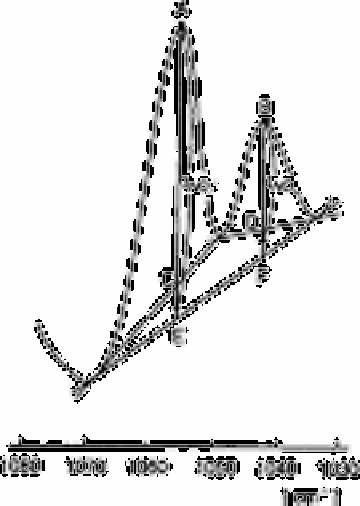
Fig. 2.34 The spectrum of the mixed solution of the coloured product and
nitrate. The base lines for the analysis are determined as follows:
I
NO3
−
=BD+BF×DF/AE+BF).I
NO2
=AC+AE×CEAE+BF). The
concentrations of the NO
2
−
=0.2mg L
−1
and NO
3
−
= 100mg L
−1
Source: Reproduced with permission from Elsevier Science [577]
Search WWH ::

Custom Search