Chemistry Reference
In-Depth Information
or the colour-development stage, phosphate, silicate, hydrogen carbonate, sulphide and
cysteine were added to nitrite determinations with the reduction step omitted; only
sulphide interfered. However it is unlikely that it interferes at the same stage in the nitrate
determination because all sulphide will have probably have been previously removed as
cadmium sulphide.
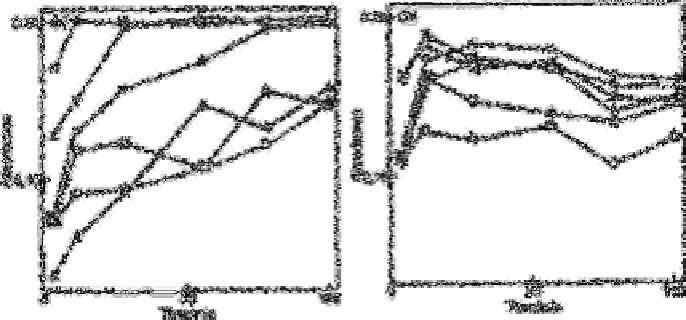
Fig. 2.25(a) shows how the absorbance, in the presence of known interferences, varies
with the time of reduction. The effect of all interfering substances is reduced on
increasing the shaking time and near theoretical yields are obtained in the presence of
high concentrations of hydrogen carbonate and cysteine after 50 and 140min,
respectively. Longer times would be necessary to overcome the problems created by
phosphate, silicate and sulphide.
The same time-dependent interference studies were conducted using spongy cadmium
and the results are shown in Fig. 2.25(b). The absorbance increased with shaking time up
to 20min, and after that it decreased slightly with time. Phosphate, hydrogen carbonate
and cysteine did not show any appreciable interferences after this time. Although
sulphide and silicate did interfere, their concentrations were much higher than those
usually encountered in natural freshwater samples.
Fig. 2.25
Plot of absorbance vs reduction time: (a) Using 0.5-0.6g of cadmium
filings; (b) Using 0.5-0.7g of spongy cadmium for a standard
solution of 0.56mg L
−1
of nitratenitrogen with the following
additions: nil; ∆ 2mol L
−1
of phosphate phosphorus;
25ml L
−1
of
silicate silicon; ×250mg L
−1
of hydrogen carbonate;
1mg L
−1
of
sulphide; • lmg L
−1
of cysteine hydrochloride
Source: Reproduced with permission from the Royal Society of
Chemistry [391]


Search WWH ::

Custom Search