Chemistry Reference
In-Depth Information
Moss and Stephen [139] determined chloride, bromide and iodide by converting them
to alkylmercury(II) halides and measurement by high performance liquid
chromatography.
Akaiwa
et al.
[140] have used ion exchange chromatography on hydrous zirconium
oxide combined with a detection based on direct potentiometry with an ion selective
electrode for the simultaneous determination of chloride and bromide in non saline
waters.
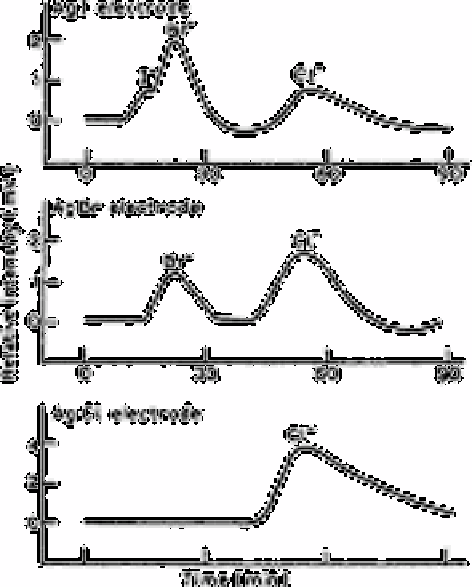
Fig. 2.23
Chromatograms of halide standards: iodide 5.0ng mL
−1
; bromide
50ng mL
−1
; chloride 2.0µg mL
−1
Source: Reproduced with permission from Elsevier Science [140]
The response of the three silver halide electrodes in the chromatography of a mixture of
iodide, bromide and chloride is shown in Fig. 2.23. The silver chloride electrode gave
poor response to iodide and bromide, and so did the silver bromide electrode to iodide.
Although the silver iodide electrode responded to all three halides, the peaks are not
sufficiently resolved and they are asymmetric. Further, there was a drift of the base line
after detection of a halide ion which was not a component of the electrode and this drift
caused disturbance in the following peak. This difficulty is eliminated by using hydrous
zirconium oxide instead of the anion exchange resin for the chromatography since it
reverses the elution order for halide ions. The silver bromide electrode is then the most
Search WWH ::

Custom Search