Chemistry Reference
In-Depth Information
The other method [229], involves the use of peroxidase, in which non-fluorescent
homovanillic acid is converted by hydrogen peroxide into the highly fluorescent 2,2
′
-
dihydroxy-3,3
′
-dimethoxybiphenyl-5,5
′
-diacetic acid:
2.23.3
Chemiluminescence methods
One method is based on the inhibiting action of this anion on the chemiluminescence of
luminol with hydrogen peroxide in the presence of copper(II) [265]. The induction period
increases with increase in the concentration of cyanide and the logarithm of the induction
period is proportional to the concentration of cyanide.
Ishii
et al.
[266] used flow injection analysis with chemiluminescence detection for
determining very low levels of cyanide in non saline waters by means of the uranine-
sodium hydroxide-didocecyldimethylammonium bromide system. The emission induced
by cyanide is efficiently sensitised by uranine in organised surfactant aggregate bilayer
vesicles solution. The method is selective for cyanide and has a detection limit of
3×10
−10
M for continuous sample flow and of 2×10
−9
M for 20µL sample injection. The
relative standard deviation was 2.1% (
n
=10) for 0.05ng of cyanide.
2.23.4
Atomic absorption spectrometry
Rosentreter and Skogerboe [267] and Razmilic [268] have described an atomic
absorption spectrometric method for the determination of sub-ppm concentrations of
cyanide in non saline waters.
Haj-Hussein
et al.
[269] have developed a flow injection method for the determination
of cyanide ion by atomic absorption spectrometry. Aqueous cyanide samples (pH 11.0)

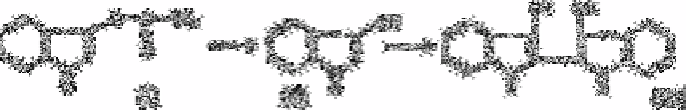
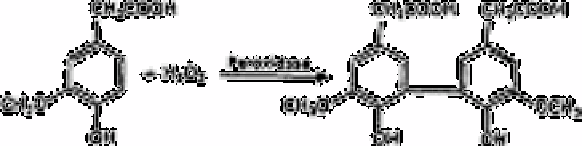

Search WWH ::

Custom Search