Chemistry Reference
In-Depth Information
Fig. 2.19
Autoanalyser method for determination of cyanide
Source: Reproduced with permission from Butterworth Press, UK
[244]
through the solution to displace cyanide, and collecting it in a glass bead concentration
column. In a second method, hydrogen cyanide was allowed to diffuse from an enclosed
solution into dilute sodium hydroxide in a dish suspended above the solution. The
separated and concentrated cyanide was determined spectrophotometrically by the
chloramine-T method. The diffusion procedure was recommended for determining low
concentrations of hydrogen cyanide.
Reagents
Sodium hydroxide, 1N
Sodium hydroxide, 0.02N
Acetic acid, 2.25N
Phosphate buffer, chloramine-T [247]
Chloramine-T, 1g 1100ml
−1
Standard cyanide solution: prepare concentrated sodium cyanide solutions, dilute to the
required concentrations and adjust to pH 5.5 with hydrochloric acid to convert to free
cyanide.
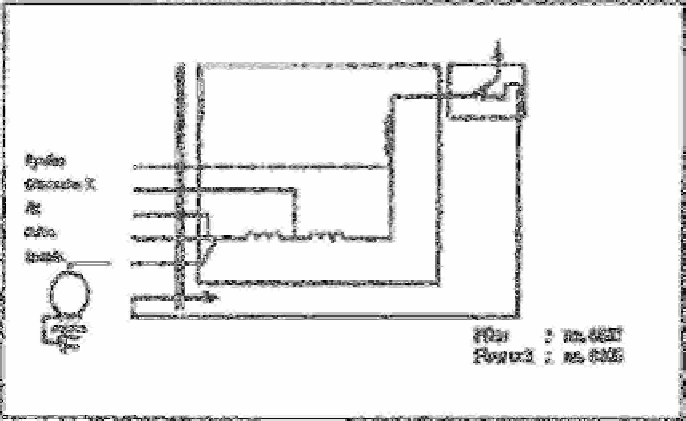
The apparatus used to isolate and concentrate hydrogen cyanide by the diffusion
principle is shown in Fig. 2.20 [248].
Search WWH ::

Custom Search