Chemistry Reference
In-Depth Information
magnesium gave poor resolutions.
Fig. 2.17
Chromatogram of orthophosphate, pyrophosphate, tripolyphosphate
and trimetaphosphate. Eluant was Tri-sulphate buffer (pH 8.2)
Source: Reproduced with permission from the American Chemical
Society [237]
Oxalate plus magnesium buffer should be freshly prepared as it is subject to
decomposition. The column should be rinsed after usage.
Calibration curves prepared by injecting an aliquot of different concentrations showed
a linear relationship with a dynamic range of 10
−4
-10
−2
mol L
−1
. The practical detection
limit was estimated for the chromatogram taking the peak height to noise level ratio at 2.
It was 0.5µg of P, 1µg of P and 3µg of P for mono-, di- and triphosphate.
With the use of a basic eluant such as ammonium or Tris buffer, it was noticed that
triphosphate was rapidly decomposed to orthophosphate and pyrophosphate during
chromatography. Fig. 2.17 shows the chromatogram of orthophosphate, pyrophosphate,
triphosphate and metaphosphate using Tris buffer as an eluant. It may reasonably be
expected that the triphosphate peak would appear later than pyrophosphate. However, no
peak appeared in such a later retention time range and, instead, two peaks which had the
same retention time as orthophosphate and diphosphate appeared. The areas of the first
and second peaks had the ratio of 1:2 indicating the mole ratio of 1:1. This suggests that
the third P-O bond of triphosphate is readily cleaved at the column head giving 1mol of
phosphate and 1mol of diphosphate. Diphosphate is stable enough to give a single peak
during chromatography. When triphosphate aqueous solution was kept at room
temperature for 1 week, significant deterioration was observed. Basic condition and the
presence of µ-Bondapak-NH might accelerate decomposition rate.
When the ionic strength of the water sample is not high, phosphate can be trapped at
the column top and be eluted with eluant buffer. By use of this concentration technique,
dilute solutions can be analysed. Orthophosphate aqueous solution (10
−4
, 10
−5
and 10
−6
mol L
−1
was pumped into the column at the rate of 1.5, 1.5 and 2.0ml min
−1
respectively.
Since too much time is required to load the water sample, a faster loading rate is
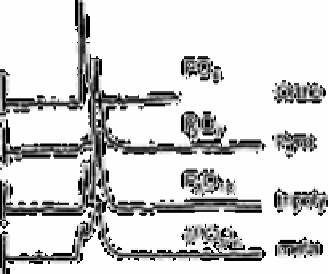
Search WWH ::

Custom Search