Chemistry Reference
In-Depth Information
(11)
(12)
Equations (1) to (12) are used in the calculation program, MECROM. Table 2.11 shows
the values corresponding to several synthetic water samples containing different
chromium concentrations. Owing to the moderately acidic pHs of these samples, the
H
2
CrO
4r
Cr(OH)
3
and Cr(OH)
4
−
contents are almost nil. The predominant species at such
pHs are Cr(OH)
2+
and HCrO
4
−
for Cr(III) and Cr(VI), respectively.
This work demonstrates that it is possible to automate studies with a non-segmented
flow system and a microprocessor. The system described provides a concentration profile
of the different chemical forms in which an element can occur in natural or artificial
samples. The most important advantage of this new technique for speciation studies are:
rapidity, easy operation and economy. The suggested system can be applied to the
speciation of other elements.
Marshall
et al.
[214] has also evaluated methods for determining the conic forms of
chromium in water while Yoshimura used ion exchanger phase absorbtiometry coupled
to flow injection analysis to determine trace levels of dichromate in water [215].
2.19.3
Atomic absorption spectrometry
In the method observed by Fukamachi
et al.
[223] and Yanagisawa [224] hexavalent
chromium (ie chromate and dichromate) is reduced by diethyldithiocarbamte to trivalent
chromium with which it forms an isobutyl methyl ketone soluble complex.
Preconcentrated chromate is then determined in the solvent extract by atomic absorption
spectrometry at 357.9nm.
Parkow
et al.
[225] have described a procedure for the differential analysis of traces of
chromium(VI) (chromate) and chromium(III) in non saline waters. The sample is filtered,
acidified and divided into three portions, one of which is left untreated while the others
are passed through a cation exchange resin and an anion exchange resin, respectively.
The three aliquots are then treated with nitric acid, evaporated and

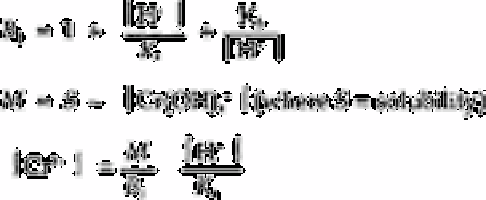

Search WWH ::

Custom Search