Chemistry Reference
In-Depth Information
iodine to iodate by potassium permanganate in sulphuric acid solution. When an aqueous
solution of iodine and permanganate is mixed with carbon tetrachloride, the iodine is
extracted into the carbon tetrachloride. If bromide is present with the aqueous
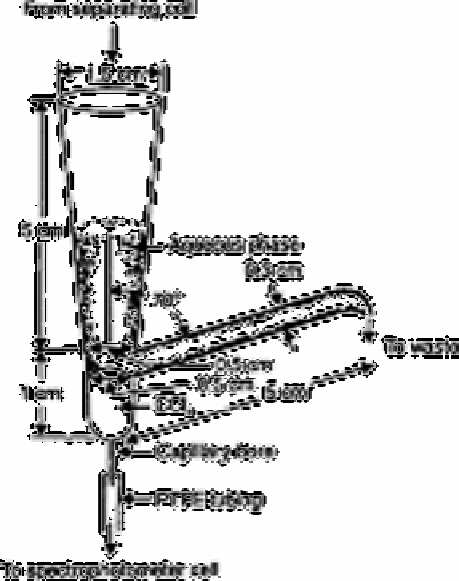
Fig. 2.5
Separator
Source: Reproduced with permission from the Royal Society of
Chemistry [122]
iodine and permanganate for a certain time at a particular temperature prior to mixing
with carbon tetrachloride, the amount of iodine remaining unreacted to iodate will
decrease linearly with increasing bromide concentration. Iodine in a carbon tetrachloride
extract can, therefore, serve as a sensitive measure of the bromide concentration in the
aqueous solution. A skilled operator is required, as the reaction temperature and the time
for each reaction step are very critical.
Pyen
et al.
[122] have described an automated version of the above manual kinetic
method. This method is accurate and precise and quicker than the manual technique (20
samples per h). The detection limit is 0.01mg L
−1
bromide.
Apparatus
The automated equipment consists of a Technicon sampler, proportioning pump,
manifold, spectrophotometer, voltage stabiliser and recorder. The spectrophotometer is
fitted with specially designed matched 50mm flow cells and 529nm filters. The sampler
Search WWH ::

Custom Search