Chemistry Reference
In-Depth Information
with 10ml of extraction solution according to the above extraction procedure. Pipette 3ml
of the organic phase and carry out the same fluorometric procedure.
The absorption spectrum of the boron-curcumin compound in isobutyl methyl ketone
obtained following the above procedure exhibits maximum absorbance at 510nm when
measured against a reagent blank solution.
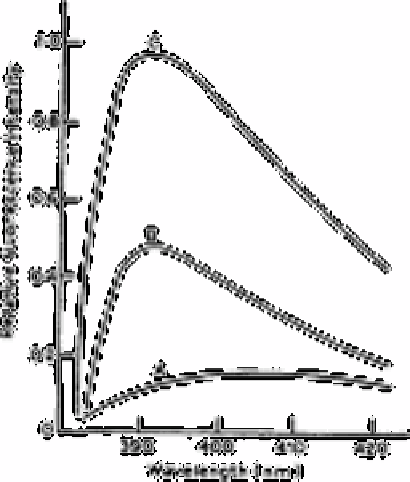
The fluorescent excitation spectrum of the boric acid dibenzoylmethane compound in
isobutyl methyl ketone against quinine sulphate solution is shown in Fig. 2.4. The
wavelength of the maximum excitation
Fig. 2.4
Fluorescence excitation spectra against quinine sulphate solution as
reference: A, reagent blank solution, B, boron-DBM, 50µg mL
−1
of
boron; and C, boron-DBM, 100µg L
−1
of boron
Source: Reproduced with permission from the Royal Society of
Chemistry [23]
radiation was 390nm. The maximum relative fluorescence intensity was measured at
400nm or by using a Kodak 2B cut-off filter (400nm cut-off).
The precision of the method for 10 replicate determinations was 0.6%. The
corresponding detection limit in the fluorometric method was 1µg L
−1
(as boron).
Interference effects in both methods are illustrated in Table 2.2. The interference of iron
at concentrations greater than 7×10
−5
mol L
−1
can be eliminated by removing iron as the
chloro complex by extraction with isobutyl methyl ketone. The total elimination of iron
was not necessary as the phosphoric acid masked the residual iron in the boric acid-
curcumin reaction.
Boron recoveries in some non saline waters were in the range 97.5-101%.
Search WWH ::

Custom Search