Biology Reference
In-Depth Information
FIGURE 16.1
Model of the type 1 secretion system. Upon recognition of a C-terminal secre-
tion signal on the secreted protein, the inner membrane complex formed by an energy-providing
ABC cassette and MFP protein interacts with the trimeric OMP. A sealed channel assembly span-
ning across the two membranes of the Gram-negative cell envelope is formed, through which the
substrate is secreted. The secreted protein is translocated in a single step, with no periplasmic
intermediate.
The secreted proteins are diverse in function and include toxins, proteases,
lipases, S-layer proteins, hemophores, bacteriocins, and others with as yet
unknown functions (
Delepelaire, 2004
). Although divergent in sequence, the
majority of proteins secreted by the T1SS have a C-terminal located secretion
signal, which is recognized by the translocation machinery; the exception is
dispersin of EAEC which is secreted to the periplasm by the Sec system before
secretion via the T1SS to the extracellular milieu (
Koronakis et al., 1989; Ghigo
and Wandersman, 1994; Sheikh et al., 2002
).
The translocating machinery of a T1SS is comprised of three proteins that
span the cell envelope, all of which are essential for secretion (
Letoffe et al.,
1996
). Two of the translocation proteins span the inner membrane (the ATP-
binding cassette [ABC] and the membrane fusion/adapter protein [MFP]),
while the final member of the translocator is an outer-membrane protein (OMP)
(
Figure 16.1
). The ABC protein possesses a nucleotide-binding domain (NBD)
fused to a transmembrane domain (TMD), and recognizes the C-terminal secre-
tion signal of the substrate molecule; as such this protein is responsible for the
specificity of the secretion machinery for the substrate molecule (
Delepelaire,
2004
). The MFP protein consists of a short cytoplasmic domain at the N-
terminus, followed by a membrane anchor and a large periplasmic domain

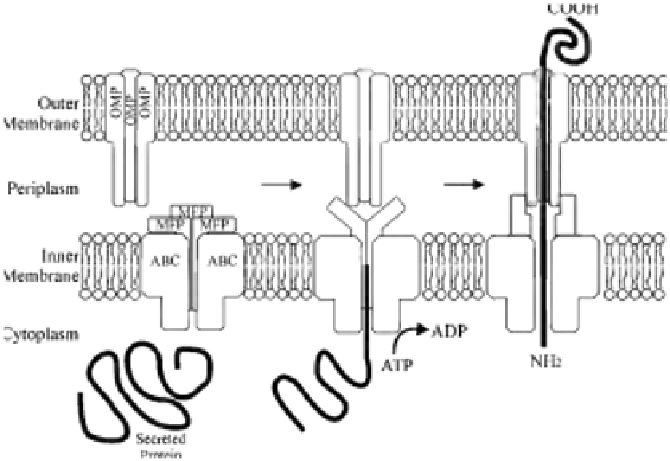
Search WWH ::

Custom Search