Chemistry Reference
In-Depth Information
At this same time there are data that arylsulfanamides may inhibit radical-chain process
of destruction. Kinetics of inhibited radical polymerization of methyl methacrylate, initiated
by dinitrile of azo - bis - isobutyric acid, was studied to evaluate inhibiting activity of
carbazolsulphonamides.
Inhibiting ability of carbazolsulphonamides was characterized by change of the rate of
methyl metacrylate polymerization in the presence of suggested compounds in the mode of
the stationary flow of the process (W
int
st
) and “gel-effect” (W
int
gel
) and also by the factor of
inhibiting (F), the value of which is proportional to the constant of the rate of inhibition.
As it is seen from kinetic parameters, given Table 20, all carbazolsulphonamides on way
or another decrease the rate of polymerization, however, induction period here is absent,
hence, these compounds are weak inhibitors. May be additional shielding effect after addition
of carbazolsulphonamides in connected with their participation in inhibition of radical
processes of CDA photodestruction.
Tinuvine -II, which, as it is known, is not an inhibitor of radical processes, appeared to
be less active stabilizer of photooxidative destruction.
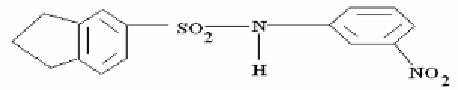
To compare the effect of “carbazole” component of sulphonamides on light stabilizing
activity there has been carried out their synthesis on the basis of coke-chemical indan (Table
21).
Table 21. Viscosity maintenance in CDA- films containing indan sulphuryl amide
additions in ultra-violet radiation
Structure
Т,
0
С
135-136
36,6
158-159
42,2
144-146
32,2
185-186
47,7















Search WWH ::

Custom Search