Chemistry Reference
In-Depth Information
It is seen from this figure that data, obtained as a result of these experiments, are
identical, that proves antioxygenic action of XXXV.
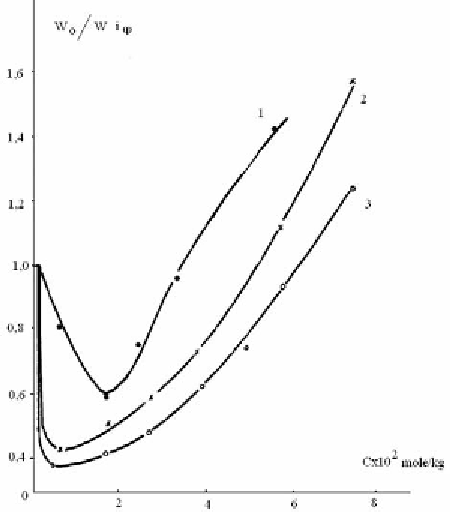
Figure 2.16. Dependence of the rate of acetic acid accumulation on additives concentration: 1 -
Tinuvine in the air; 2 - XXXV - in the air; 3 - XXXV in vacuum.
Comparisons of XXXV with well-known antioxidant - Tinuvine over the wide range of
concentrations have been carried out in further experiments. Data of these experiments are
given in Figure 2.16, from which it is seen that XXXV, similar to Tinuvine, accelerates
phototransformation in the range of large concentrations, and in the region of small
concentrations it decelerates this process. Low value of maximum concentration of XXXV
may be connected with the fact that reaction proceeds in noncrystal regions of polymer,
which are more available for additives. It should be noted here that XXXV acts as antioxidant
at much lower concentrations than Tinuvine.
The fact that XXXV displays light-protective activity, decelerating CDA destruction at
ultraviolet irradiation not only in vacuum (Figure 2. 16, curve 3), but in the air (Figure 2.16,
curve 2), moreover, curves 2 and 3 have the character close to curve 1, that undoubtedly
testifies in favour of XXXV action not only as ultraviolet absorber, but also as an antioxidant.
Possibility to decelerate the rate of CDA photodestruction by the products of inhibitors
transformation is important, too.
It is known that PAC plays the role of inhibitors of radical-chain processes, specifically,
polymerization [177]. In all probability, interaction of PAC molecule with being formed
radicals in CDA at irradiation takes place. Reaction between radicals and inhibitor with
formation of completely inactive products may probably proceed here:
Р
*
+ I
n
К1
→ inert products

Search WWH ::

Custom Search