Chemistry Reference
In-Depth Information
absorption in post-effect at quadratic break of oxidation chains in the presence of dark process
of initiation is described by the equation [174]:
[∆O
2
]=Kn[RH]/Kr*ln((1+ξbh(t/ τ st)/1+bh(t/ τ st))+Wst*t
(1)
where, K
n
and K
r
- constants of rates of reactions of continuation and break of oxidation
chains, [RH] - concentration of reactive part of monomeric units, τ
st
=1/K
r
[R*O
2
]
st
- life-time
of radicals in stationary mode of oxidation, [R*O
2
]
st
- concentration of radicals in stationary
mode, ξ=W
0
/W
st
(where, W
0
and W
st
- rates of oxidation at the initial moment of time after
switching off the light and in stationary mode of dark oxidation, correspondingly.
Provided, that t » t
st
, equation (1) assumes the form:
[∆O
2
]=Kn[RH]/Kr*ln((1+ξ)/2)+Wст*t
(2)
According to the equation (2), experimental data in [∆O
2
] - t plot of large t is described
by strait line, tangent of slope angle of which is equal to W
st
, and cutting on Y-axis is equal to
[∆O
2
]=Kn[RH]/Kr*ln((1+ξ)/2)
(3)
Substituting experimental value of ξ=5,3 into the equation (3) important parameter,
characterizing chain photooxidation has been found K
n
[RH]/К
r
= 0,9·10
-5
mole/kg.
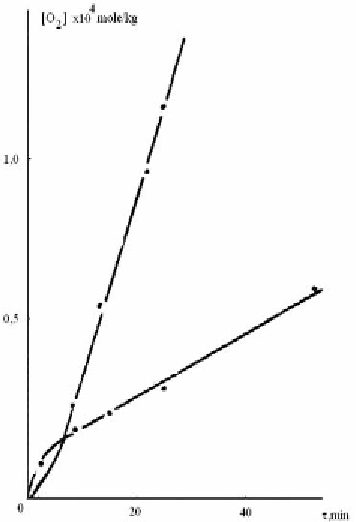
Figure 2.6. Kinetic curves of oxygen absorption at irradiation (1) and after irradiation (2) of CDA by
the light with wave length of 253,7 nm and intensity 1-0,5·10 quant/cm”#s at 298°K and pxygen
pressure 150 T. 2-theoretical curve of photochemical aftereffect, calculated according to the equation
3.1 at Kn[RH]/Kr=o,9-10”5 mole/kg, y=5,3; T=300s; WTeM=1,69-10”8 mole/kg·s.

Search WWH ::

Custom Search