Chemistry Reference
In-Depth Information
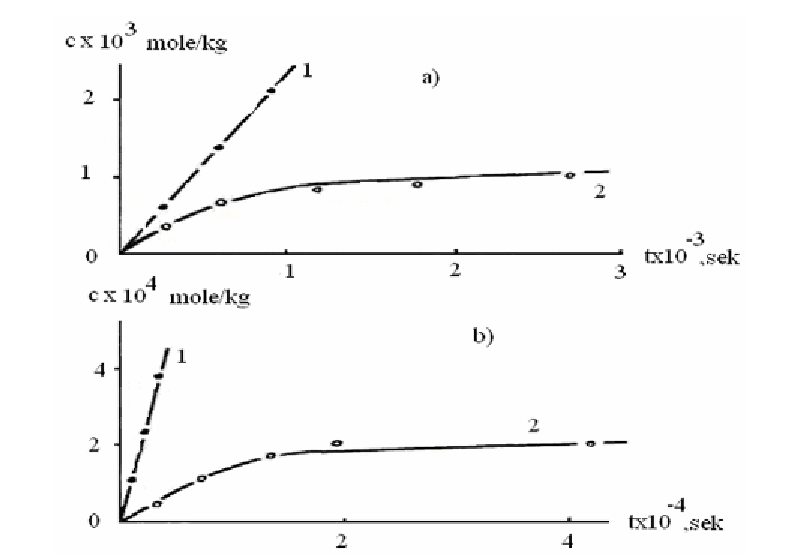
Figure 2.4. Kinetic curves of accumulation of HCl (1) and radicals (2) at CA films irradiation with
addition of 0,01 mole/kg of TCMP; light intensity is: a) 90·13, b) 4,4·1013 quant/cm·sec.
This shows that the rate of observed radicals formation is proportional to I
1,5
. So, the
reaction of observed radicals formation has the same order, according to light intensity, as the
reaction of acetic acid formation. That is why formation of observed radicals and acetic acid
proceeds in parallel reactions.
It is interesting to note, that at low light intensities the rate of formation of observed
radicals is much lower than W
in
(Figure 2.4 b). This indicates the presence of high-active
radicals in the system with large constants of the destruction rate.
Experiments at lowered temperatures were carried out for discovering these radicals. EPR
spectrum, being superposition of singlet and triplet signals (Figure 2.5 a), is registrated at CA
irradiation at 20°C. Warming of the samples destroys active radicals. Only stable singlet 1,6
mT wide remains here (Figure 2.5 b). Analogous singlet is observed at TCMP photolysis at
25°C. Difference of the first and second spectra gives EPR spectrum of radicals, destructed at
warming up (Figure 2.5 b): it is triplet with STV splitting by 2,9 mT.
Singlet 1,6 mT wide being observed at photolysis and radiolysis of cellulose and its
derivatives is attributed to radicals with the chain of conjugation: these are alkyl and polyenyl
radicals [148].
Triplet 2,5-3,0 mT wide is attributed to radicals, obtained by breaking of hydrogen atom
from carbon in positions 2, 3 and 5: these are hydroxylic radicals in cellulose and acetyl alkyl
radicals in cellulose diacetate.
Search WWH ::

Custom Search