Chemistry Reference
In-Depth Information
It should be noted that formation of ketene and acetic acid is not observed at photolysis of
glucose and cellulose.
So, one may come to a conclusion: there is no unity of views of researchers regarding
photodestruction of cellulose and its derivatives, and for better understanding the mechanism
of phototransformation it is necessary to take into account that while studying kinetics of
phototransformation one should consider the following factors: effect of supermolecular
structure and initialing or inhibiting action of impurities.
2.2.
A
BOUT THE
M
ECHANISM OF
P
HOTOOXIDATIVE
D
ESTRUCTION
OF
C
ELLULOSE
A
CETATE
As the main product of CA photodestruction is acetic acid, formed as a result of the break
of bonds C-O-C at carbon atoms in positions 2, 3 or 6 [2], it is suggested that break of these
bonds run in direct photolysis with splitting radical AcO* out, which in further reactions
breaks atom H from polymer and is transformed into acetic acid [2].
However, being formed acetyl radical (AcO*) is not stable and easily decomposes with
formation of CO
2
[160]. That is why, kinetics of accumulation of acetic acid and radicals at
initial stages of cellulose diacetate (CDA) photolysis has been studied for full understanding
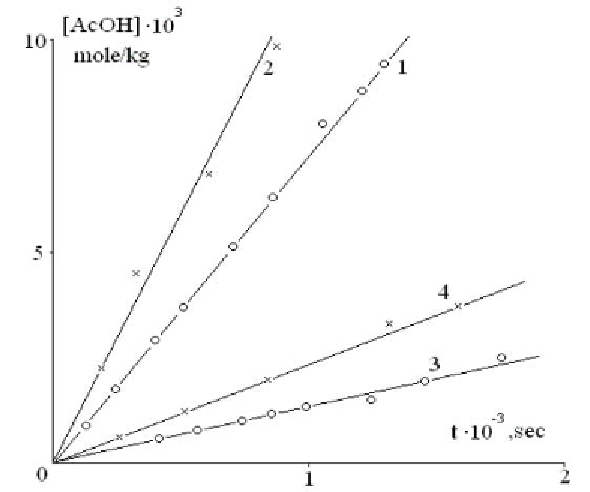
of the mechanism of glucoside bonds breakage and formation of acetic acid. Typical curves
of acetic acid accumulation at CA films irradiation are given in Figure 2.1.
Figure 2.1. Kinetic curves of acetic acid accumulation at CA films irradiation at 25°C in vacuum (1,3)
and in the air (2,4); light intensity is 10·1014 (1,2) and 3,6·1014 quant/cm2·sec (3,4).
As it is seen from Figure 2.1 constant rate of acetic acid accumulation is stated soon after
the beginning of irradiation. The rate of the process in the presence of oxygen of the air is

Search WWH ::

Custom Search